Ion sieve for selectively extracting lithium and application thereof
A technology for ion sieving and lithium extraction, applied in other chemical processes, chemical instruments and methods, etc., can solve the problems of unfavorable industrial application, short life of adsorbent, large dissolution loss, etc., and achieve low production cost, low price and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
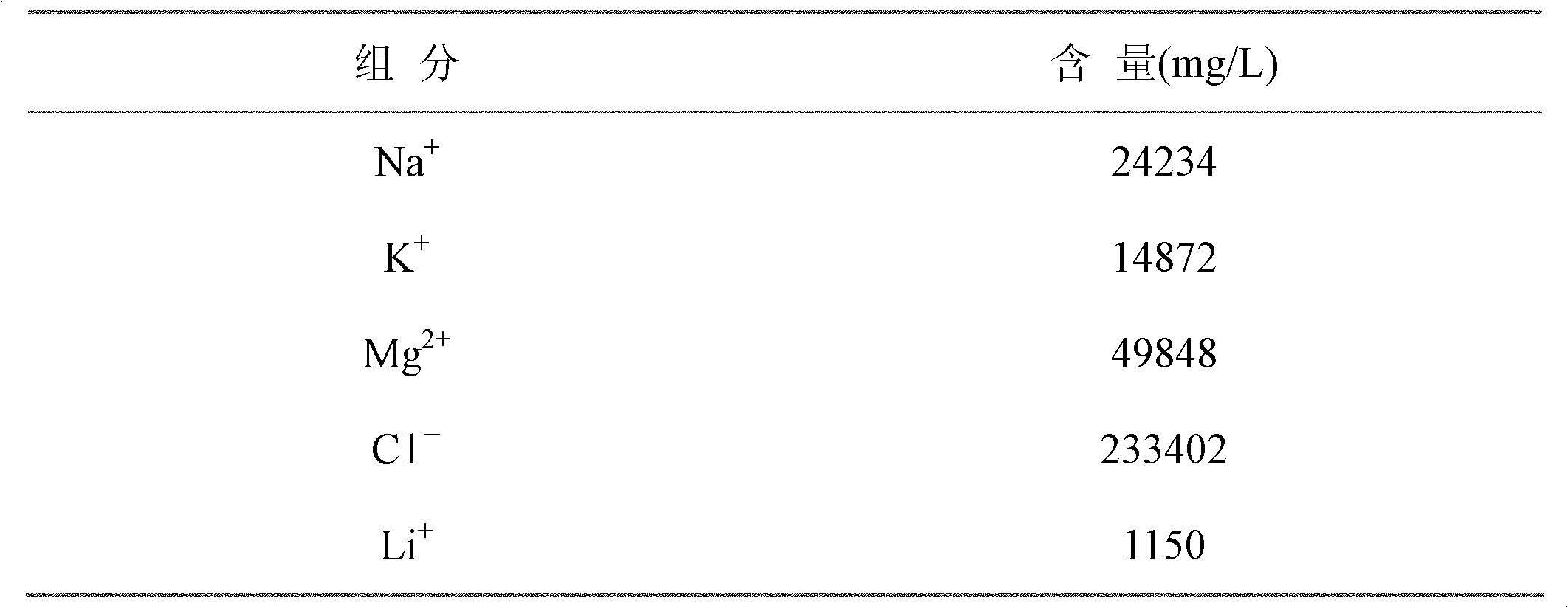
[0015] 100g Li 4 Ti 5 o 12 The ion sieve is placed in 50L of a salt lake brine. The main components and contents of the brine are shown in the following table:
[0016]
[0017] will be mixed with 100gLi 4 Ti 5 o 12 The 50L salt lake brine of the ion sieve was heated and stirred, and the temperature of the solution was controlled at 80°C; 50mL of 10% hydrazine aqueous solution was slowly added dropwise, stirred and reacted for 1h, and then filtered, the Li in the brine + Enter Li 4 Ti 5 o 12 In the ion sieve, the solid phase is gradually transformed into Li 7 Ti 5 o 12 , Li in solution + The concentration of Li was reduced to 1.09g / L, Li 4 Ti 5 o 12 The adsorption capacity of ion sieve to Li is about 30mg / g; 7 Ti 5 o 12 Place in 1L of 10% NaCl solution for heating and stirring, control the temperature of the solution at 40°C, slowly add 25mL of 25% hydrogen peroxide dropwise, stir and react for 1h and then filter, Li 7 Ti 5 o 12 Li in + Into the NaCl so...
Embodiment 2
[0019] 200g Li 4 Ti 4.98 Zr 0.02 o 12 The ion sieve material was loaded into an ion exchange column, and a mixed solution was prepared with 90 mL of 10% hydrazine aqueous solution and 20 L of salt lake brine. The main components and contents of the salt lake brine were the same as in Example 1. Pass the mixed solution through the ion-exchange column at a speed of 100mL / min; after the ion-exchange column leaks through, wash the ion-exchange column with distilled water, and then use 2L of a solution containing 30g / LNaCl and 6g / L hydrogen peroxide at a rate of 100mL / min Speed through the ion exchange column, oxidative desorption to obtain Li + Concentration is 6.46g / L solution 1L, Li 4 Ti 4.98 Zr 0.02 o 12 The Li adsorption capacity of the ion sieve is about 32.3 mg / g.
Embodiment 3
[0021] 20gLi 3.95 Nb 0.01 Ti 5 o 12 The ion sieve is placed in the brine chamber of the electrodialysis device, and 2L of a certain salt lake brine is added. The composition and content of the salt lake brine are shown in the following table:
[0022]
[0023] Add 100mL of 30g / L NaCl solution into the lithium salt chamber in the electrodialysis device, and the brine chamber and the lithium salt chamber are separated by an anion exchange membrane; with graphite as the anode, Li 3.95 Nb 0.01 Ti 5 o 12 As the cathode, a voltage of 0.8V is applied across the electrodes, and after maintaining for 15h, the Li in the brine chamber + Concentration decreased to 259mg / L, Mg 2+ The concentration is about 17993.8mg / L, Li 3.95 Nb 0.01 Ti 5 o 12 to Li + The adsorption capacity is about 24.1mg / g, for Mg 2+ The adsorption capacity is about 0.62mg / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com