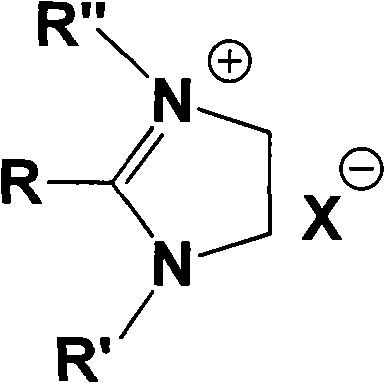
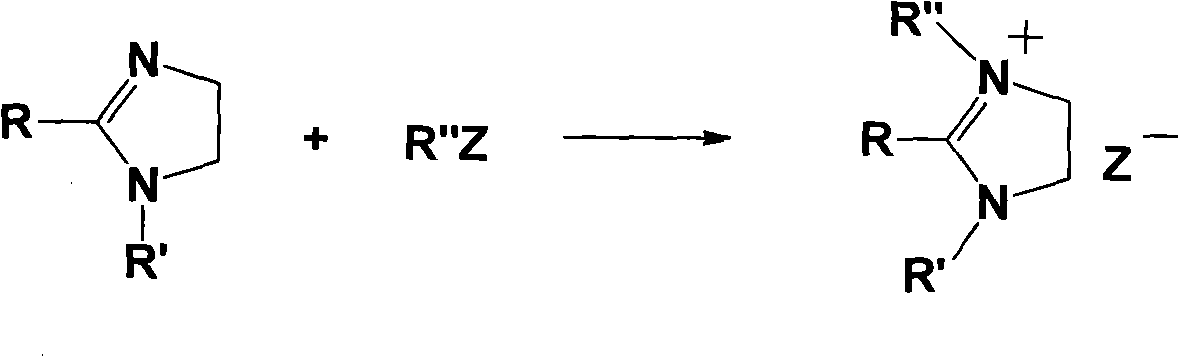
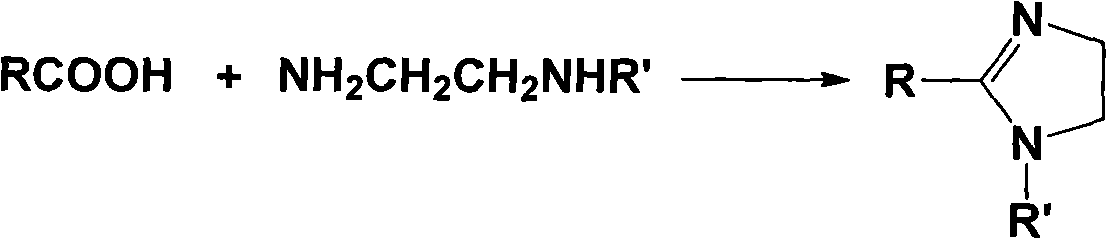Imidazoline ionic liquid and preparation method and application thereof
A technology of ionic liquids and imidazolines, which is applied in the field of imidazolines ionic liquids and their preparation, achieving the effects of low preparation cost, mild synthesis conditions and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 11-Butyl-2-methyl-3-ethyl imidazoline hexafluorophosphate
[0043] (1) Synthesis of 1-butyl-2-methylimidazoline
[0044] (a) Add 1.7 moles of acetic acid to a 250mL three-necked round-bottomed flask equipped with a thermometer, a spherical condenser and a constant pressure dropping funnel, slowly add 0.8 moles of N-butylethylenediamine dropwise, and the reaction solution turns light brown . After the dropwise addition, change to a distillation device to steam out the water and excess acetic acid generated by the reaction. Distillation under reduced pressure then obtains colorless viscous liquid 1-butyl-2-methylimidazoline;
[0045] (b) Add 0.4 moles of 1-butyl-2-methylimidazoline and 0.6 moles of finely ground calcium oxide powder in step (a) to a 250mL three-neck round bottom flask, heat to 240°C, and reflux for 12 hours , Distilled and purified to obtain deacidified 1-butyl-2-methylimidazoline.
[0046] (2) Synthesis of 1-butyl-2-methyl-3-ethyl imidazoline...
Embodiment 2
[0052] Example 21-Butyl-2-methyl-3-butylimidazoline tetrafluoroborate
[0053] (1) Synthetic 1-butyl-2-methylimidazoline according to Example 1
[0054] (2) Synthesis of 1-butyl-2-methyl-3-butyl imidazoline chloride salt
[0055] Get 0.3 moles of 1-butyl-2-methylimidazoline and dissolve it in 180 mL of acetonitrile, then add 0.4 moles of chlorobutane, reflux for 36 hours, evaporate the solvent and excess chlorobutane under reduced pressure to obtain 1-butyl- 2-Methyl-3-butylimidazoline chloride;
[0056] (3) Dissolve 0.3 mol of 1-butyl-2-methyl-3-butylimidazoline chloride salt obtained in step (2) in 200 mL of water, and add 0.4 mol of NaBF at a solution temperature of 25°C 4 , reacted for 3 hours, added 300mL chloroform for extraction, dried the organic phase, and removed the chloroform solvent under reduced pressure to obtain ionic liquid 1-butyl-2-methyl-3-butylimidazoline tetrafluoroborate, with a purity of ≥ 98%.
[0057] The structural formula is as follows:
[0058...
Embodiment 3
[0060] Example 31-Butyl-2-methyl-3-butylimidazoline trifluoromethanesulfonamide salt
[0061] (1) Synthetic 1-butyl-2-methylimidazoline according to Example 1
[0062] (2) Synthesis of 1-butyl-2-methyl-3-butyl imidazoline iodide salt
[0063] Dissolve 0.3 mole of 1-butyl-2-methylimidazoline in 50-250 mL of acetonitrile, add 0.4 mole of iodobutane, reflux for 36 hours, evaporate the acetonitrile solvent and excess iodobutane under reduced pressure to obtain 1-butane Base-2-methyl-3-butylimidazolinium iodide salt;
[0064] (3) Dissolve 0.3 mole of 1-butyl-2-methyl-3-butylimidazoline iodide salt obtained in step (2) in 200 mL of water, and add 0.4 mole of Ag(CF 3 SO 3 ), reacted for 3 hours, removed water, added 300mL dichloromethane for extraction, dried the organic phase, and removed the organic solvent under reduced pressure to obtain ionic liquid 1-butyl-2-methyl-3-butylimidazoline trifluoromethylsulfonamide Salt, purity ≥98%.
[0065] The structural formula is as follow...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com