Preparation method of O-hydroxypropyl-N-alkylated chitosan surfactant
A technology of surfactant and chitosan, which is applied in chemical instruments and methods, chemical/physical processes, transportation and packaging, etc., can solve the problems of single product and lack of system, achieve good surface activity and save reaction raw materials and the effect of time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 2g of chitosan into 5mL of 33wt% NaOH and soak for 2 hours and then freeze-dry; mix the dried alkaline chitosan with 20mL of isopropanol and stir for 30min, add 1mL of catalyst tetramethylammonium hydroxide and 20mL of propylene oxide After stirring at room temperature for 60 minutes, the temperature was raised to 60°C for 8 hours (the molar ratio of chitosan, catalyst, isopropanol and propylene oxide = 1:0.5:22:24); the precipitation was allowed to settle, the upper solvent was decanted, and 20mL distilled water was added (The molar ratio of chitosan and distilled water = 1:95) Stir until it is completely dissolved, centrifuge at 4000r / min for 10min, take the supernatant and spin-evaporate at 80℃, remove the remaining isopropanol and propylene oxide, and finally get a purer An aqueous solution of alkaline O-hydroxypropyl chitosan. The preparation method of O-hydroxypropyl chitosan in Example 2 is the same as this.
[0020] The aqueous solution of O-hydroxypropyl chi...
Embodiment 2
[0022] According to the method described in Example 1, O-hydroxypropyl chitosan was obtained.
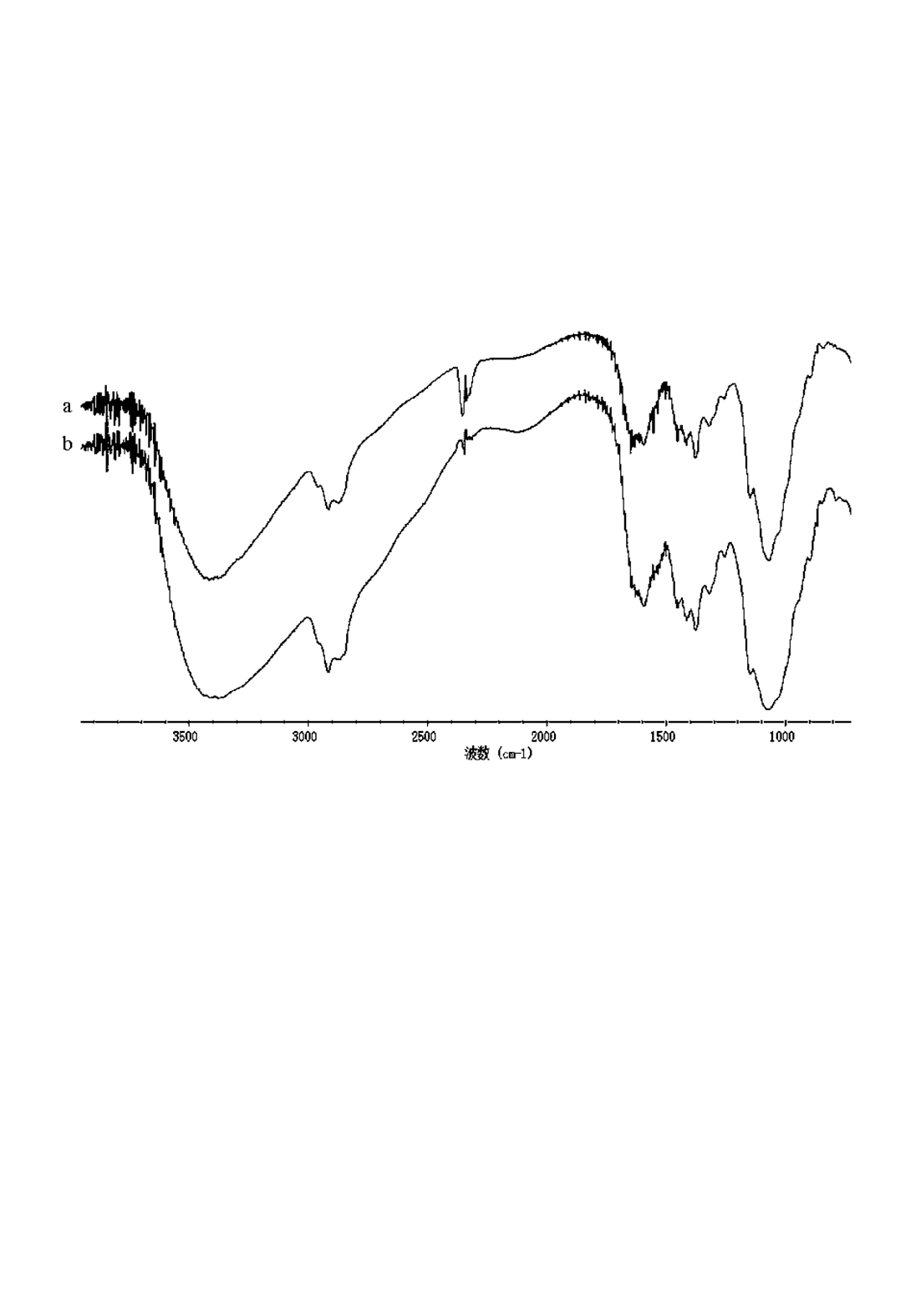
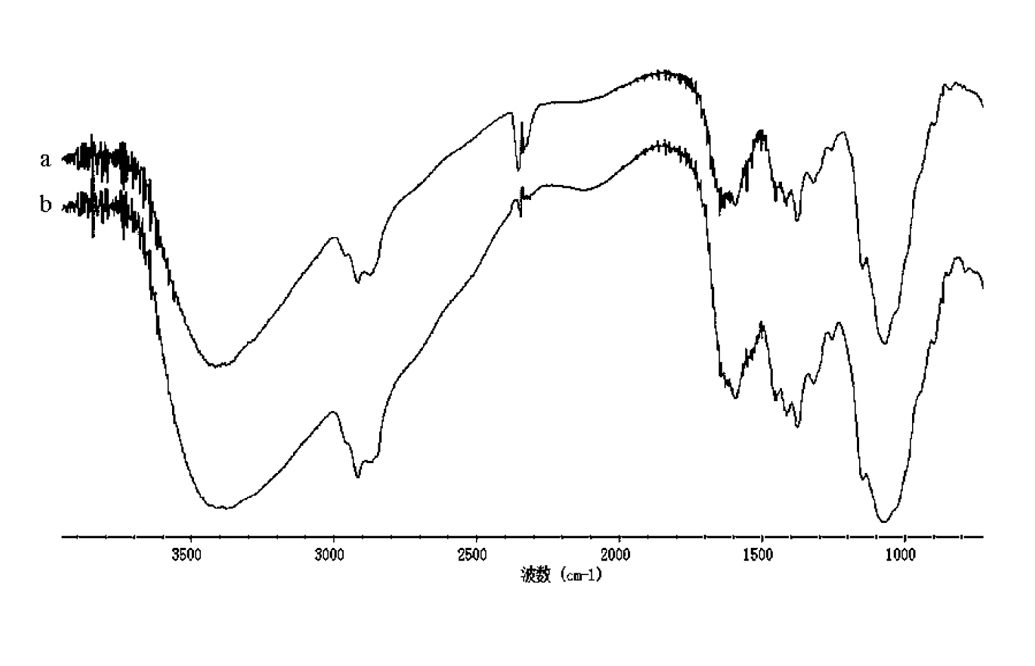
[0023] Stir the aqueous solution of O-hydroxypropyl chitosan at 40°C for 30 minutes, add 5 mL of hexadecyl bromide dropwise and increase the temperature to 80°C, add 0.06 g of cetyltrimethylammonium bromide, and stir for 8 hours. (The molar ratio of chitosan, hexadecane bromide and phase transfer catalyst = 1:1.4:0.014), cool after the reaction, and neutralize with dilute hydrochloric acid to pH 7 under stirring; add 60 mL of acetone to the reactor to cause precipitation After the precipitation no longer increases, suction filter, wash the filter cake with methanol-water (volume ratio 85:15), absolute ethanol, and dry 1.02g of white or yellow solid. The measured HLB value is 5.33, the critical glue The surface tension of the aqueous solution at a beam concentration of 0.18g / L is 24.81mN / m; it is easy to foam and has good foam stability. The infrared spectrum is measured as figure 1 A...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com