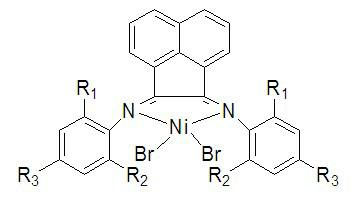
Alpha-diimine nickel (II) olefin polymerization catalyst as well as preparation method and application thereof
A technology for the polymerization of nickel diimide and olefin, which is applied in the polymer field and can solve problems such as affecting the development of ethylene polymerization catalysis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Synthesis of 2-methyl-4-phenylaniline: Palladium acetate (0.06mmol, 0.0135g), phenylboronic acid (4.5mmol, 0.5487g), 4-bromo-2-methylaniline (3mmol, 0.5582g) and potassium carbonate (6mmol, 0.8293g) were added to 5mL of polyethylene glycol-400 solvent, reacted at room temperature for 24 hours, extracted with ether after the reaction, the extract was dried with anhydrous magnesium sulfate, filtered and concentrated Afterwards, 0.5122 g of 2-methyl-4-phenylaniline was obtained by silica gel column chromatography, and the yield was 93.16%. 1 H NMR (400MHz, CDCl 3 ): δ=2.28(s, 3H), 3.69(s, 2H), 6.79(d, J = 8.0Hz, 1H), 7.34(m, 3H), 7.46(m, 2H), 7.61(m, 2H); 13 C NMR (400 MHz, CDCl 3 ): δ=17.47, 115.18, 122.48, 125.58, 126.09, 126.38, 128.56, 129.15, 131.57, 141.27, 144.03.
[0026] (2) Ligand (4-Ph-2-MePh) 2 Synthesis of DABAn: 0.4581g (2.5mmol) of 2-methyl-4-phenylaniline and 0.2277g (1.25mmol) of acenaphthenequinone were dissolved in 20mL of anhydrous methanol, a...
Embodiment 2
[0031] (1) Synthesis of 2-ethyl-4-phenylaniline: palladium acetate (0.06mmol, 0.0135g), phenylboronic acid (4.5mmol, 0.5487g), 4-bromo-2-ethylaniline (3mmol, 0.6002g) and potassium carbonate (6mmol, 0.8293g) were added to 5mL polyethylene glycol-400 solvent, reacted at room temperature for 24 hours, extracted with ether after the reaction, the extract was dried with anhydrous magnesium sulfate, filtered and concentrated Afterwards, 0.5364 g of 2-ethyl-4-phenylaniline was obtained by silica gel column chromatography, and the yield was 90.64%. 1 H NMR (400MHz, CDCl 3 ): δ = 1.34(t, 3H), 2.62(q, J =7.6Hz, 2H), 3.68(s, 2H), 6.78(d, J = 8.0Hz, 1H), 7.34(m, 3H), 7.44(m, 2H), 7.58(m, 2H); 13 C NMR (400 MHz, CDCl 3 ): δ=13.03, 24.14, 115.65, 125.46, 126.11, 126.44, 127.18, 128.24, 128.57, 131.77, 141.45, 143.45.
[0032] (2) Ligand (4-Ph-2-EtPh) 2 Synthesis of DABAn: 0.4931g (2.5mmol) of 2-ethyl-4-phenylaniline and 0.2277g (1.25mmol) of acenaphthenequinone were dissolved in 20...
Embodiment 3
[0037] (1) Synthesis of 2,6-dimethyl-4-phenylaniline: palladium acetate (0.06mmol, 0.0135g), phenylboronic acid (4.5mmol, 0.5487g), 2,6-dimethyl-4- Bromo-aniline (3mmol, 0.6002g) and potassium carbonate (6mmol, 0.8293g) were added to 5mL of polyethylene glycol-400 solvent, reacted at room temperature for 24 hours, extracted with ether after the reaction, and the extract was washed with anhydrous sulfuric acid Dried over magnesium, filtered, concentrated and separated by silica gel column chromatography to obtain 0.5386 g of 2,6-dimethyl-4-phenylaniline. The yield was 91.01%. 1 H NMR (400MHz, CDCl 3 ): δ=2.15(s, 6H), 3.54(s, 2H), 7.13(m, 3H), 7.30(m, 2H), 7.47(m, 2H); 13 C NMR (400 MHz, CDCl 3 ): δ=17.37, 121.91, 125.99, 126.43, 126.95, 128.52, 130.92, 141.43, 142.24.
[0038] (2) Ligand (4-Ph-2,6-MePh) 2 Synthesis of DABAn: 0.4933g (2.5mmol) of 2,6-dimethyl-4-phenylaniline and 0.2277g (1.25mmol) of acenaphthoquinone were dissolved in 20mL of anhydrous methanol, and 0.2mL ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| Branching factor | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com