Amphiphilic prodrug of 7- ethyl-10-hydroxycamptothecin and preparation method thereof
A hydroxycamptothecin and amphiphilic technology, applied in the field of amphiphilic drug precursors and their preparation, can solve problems such as slow drug release, achieve high drug loading, improve stability, and overcome the problem of low drug loading. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
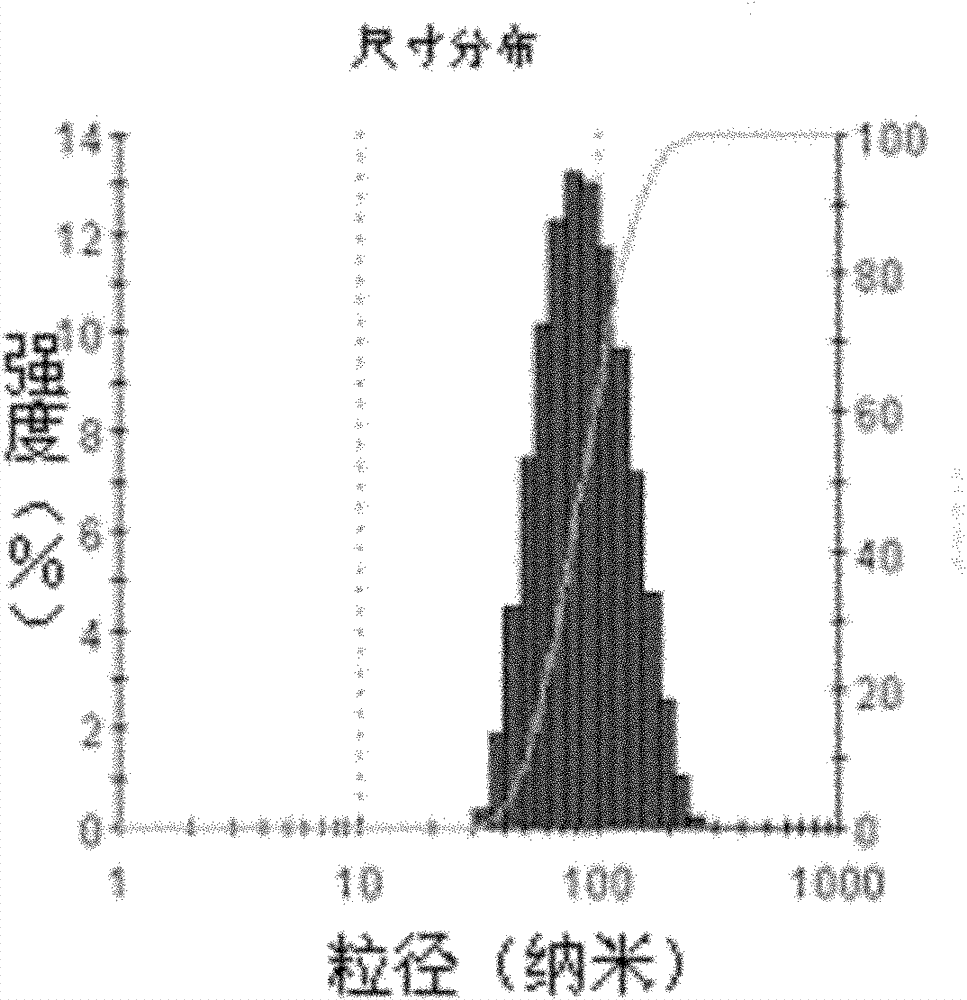
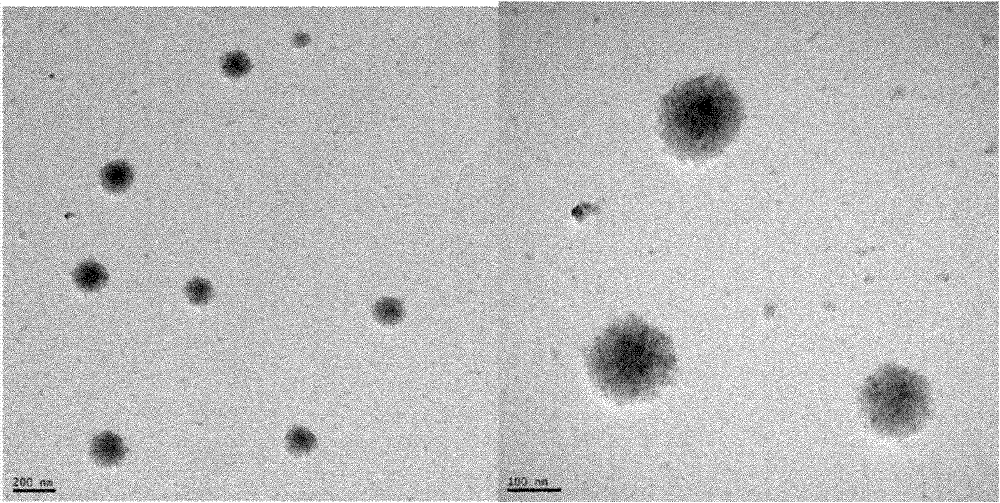
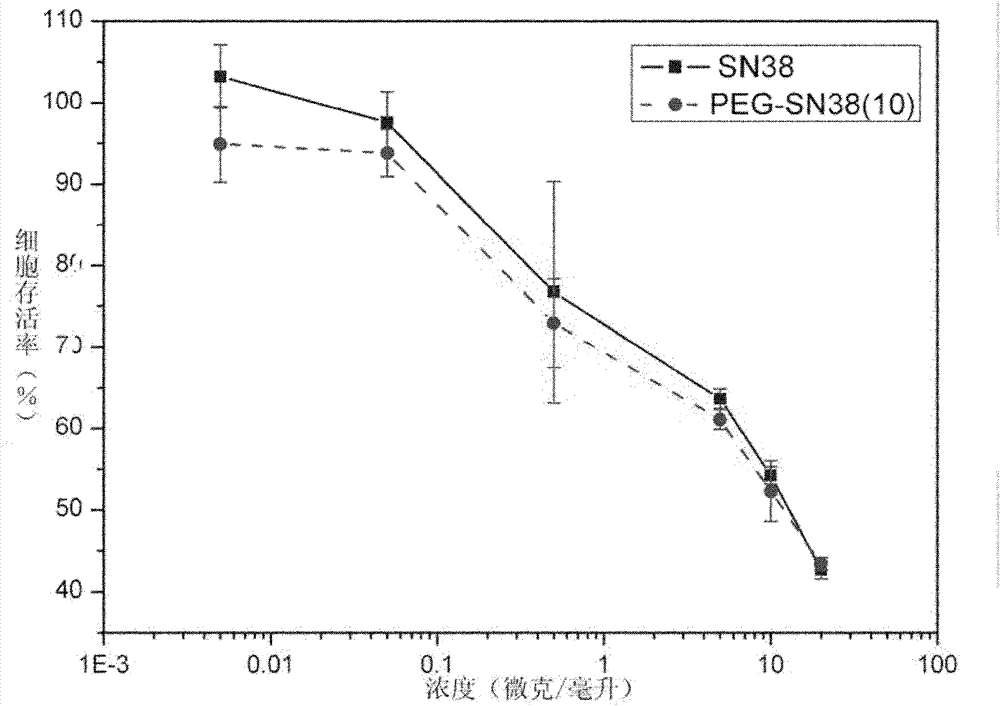
Image
Examples
Embodiment 1
[0053] The synthesis of the bond PEG-SN-38 (10) that the 10-position hydroxyl of embodiment 1PEG is connected with SN-38
[0054] (1) Synthesis of PEG-SH
[0055] Mix polyethylene glycol monomethyl ether acrylate (Mn=480, 10.8g) with ethanedithiol (10g), add 0.5ml triethylamine as a catalyst, stir overnight at room temperature, extract with water, and extract the water extract with After washing with n-hexane, the water was removed by rotary evaporation, and the 1H-NMR (400M, CDCl 3 ): δ(ppm) 4.06(t), 3.469(br), 3.450(t), 3.194(s), 2.599(m), 2.471(t), 1.604(t), indicating that the PEG with the structure shown in formula a was obtained -SH (8 g, 80% yield).
[0056] (2) Reaction of SN-38 and methacrylic anhydride
[0057] SN-38 (1g) and methacrylic anhydride (1ml) were added to 10ml of anhydrous pyridine, reacted for 5 hours, then introduced into petroleum ether for precipitation, and suction filtered to obtain the product (1.1g, yield 94%).
[0058] 1H-NMR (400MHz, CDCl 3...
Embodiment 2
[0065] The synthesis of the bond PEG-SN-38 (20) that the 20-position hydroxyl of embodiment 2PEG is connected with SN-38
[0066] (1) Protection of the 10-hydroxyl group of SN-38
[0067] Di-tert-butyl dicarbonate (Boc 2 O, 2 grams) and SN-38 (2 grams) were mixed in dichloromethane (100 milliliters), and excess pyridine (10 milliliters) was added as a catalyst, stirred until the system became clear, and then washed with 1 mol / liter of aqueous hydrochloric acid , dried over anhydrous sodium sulfate, and dried by rotary evaporation to obtain 3 g of the product, with a yield close to 100%.
[0068] product by 1 H-NMR (400MHz, CDCl 3 ) detection shows that it is a compound of structure shown in formula d.
[0069] (2) Acrylylation of the 20-position hydroxyl of the compound of structure shown in formula d
[0070] Add SN-38 with the 10-position hydroxyl protected and acryloyl chloride in anhydrous dichloromethane at a molar ratio of 1:1, use 2 times the molar amount of trieth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com