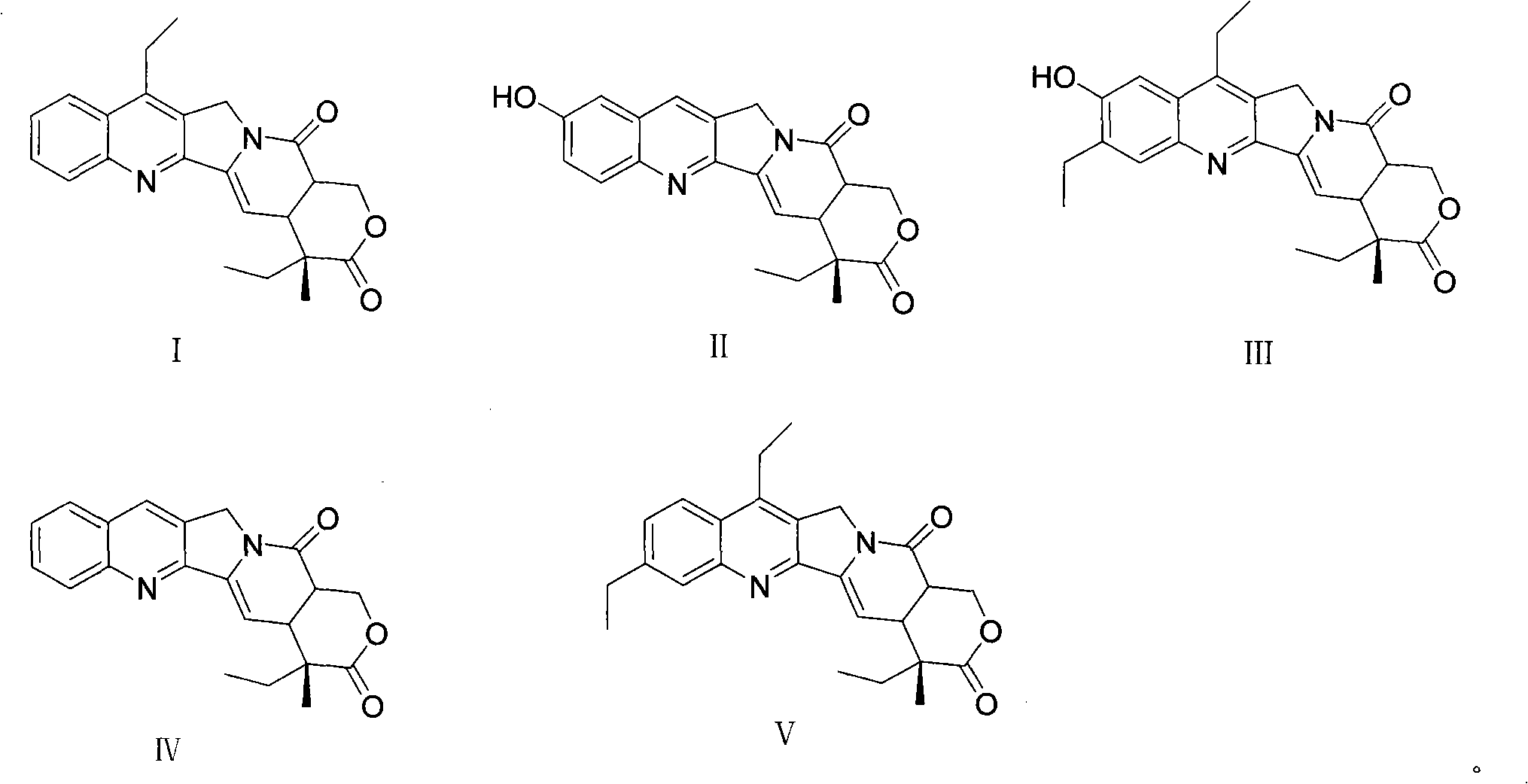
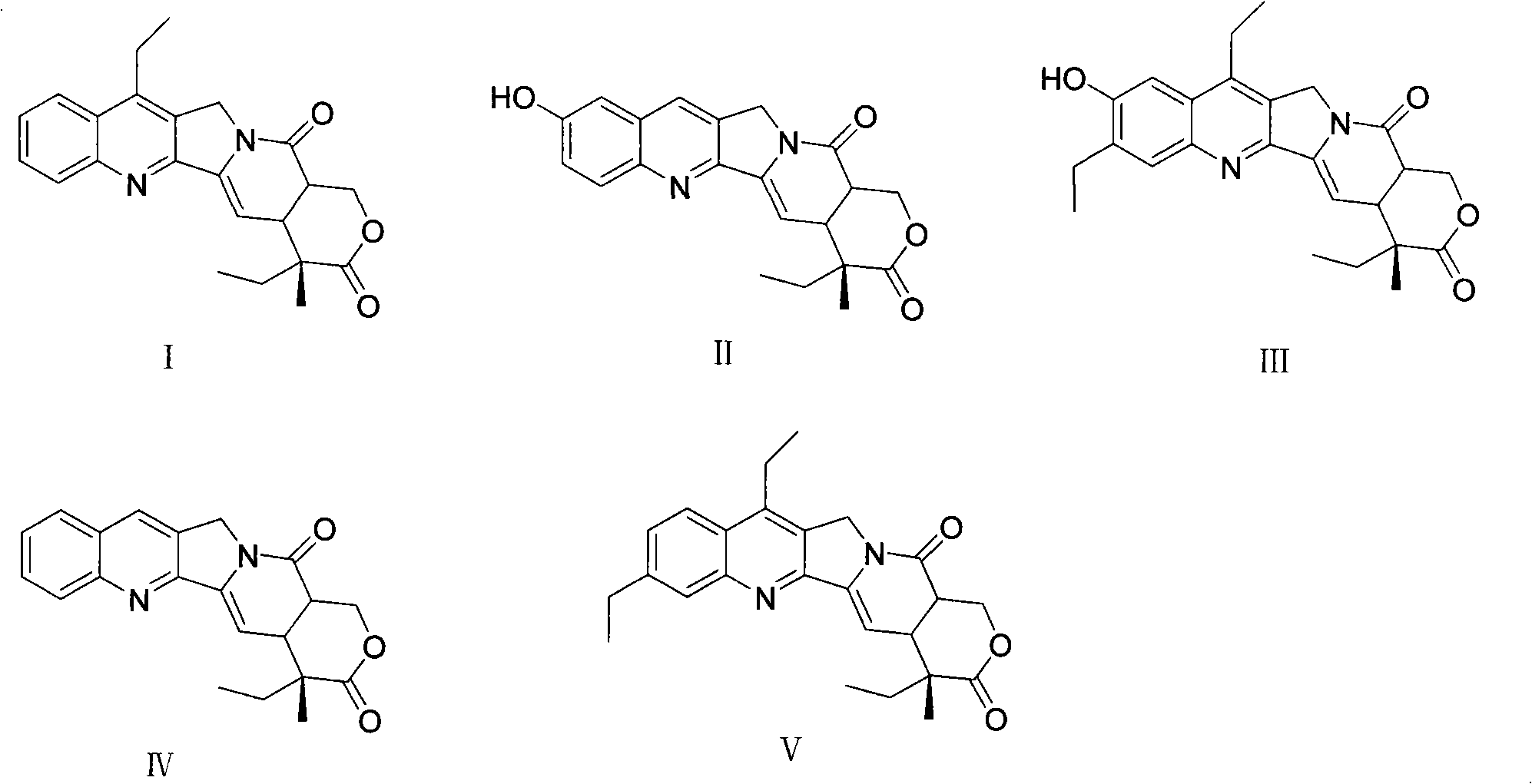
Method for producing irinotecan
A technology of irinotecan and production method, applied in the production field of irinotecan, can solve the problems of complex operation process, high production cost, long production cycle and the like, and achieve the effects of cost reduction, short reaction cycle and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0028] Example 2 Purification raw material SN38 (7-ethyl-10-hydroxycamptothecin)
[0029] 1. Transfer 2000g of SN-38 crude product and 200L methanol to the heating kettle of the cold and hot reaction kettle, heat and reflux for 1.5h, then pump it into the cooling kettle to cool to room temperature, filter with suction, distill the filtrate to recover methanol, and use the filter residue in the next step.
[0030] 2. Transfer the filter residue from the previous step and 200L acetic acid to the heating kettle of the cold and hot reaction kettle, heat and reflux until all the solids are dissolved, and then suction filter to the cooling kettle to cool to room temperature, suction filter, distill the filtrate to recover acetic acid, and use the filter residue for the next step.
[0031] 3. Transfer the filter residue from the previous step and 200L ethanol to the heating kettle of the cold and hot reaction kettle, heat until all the solids are dissolved, pump it into the cooling ke...
Embodiment 3
[0032] Embodiment 3 condensation obtains irinotecan crude product
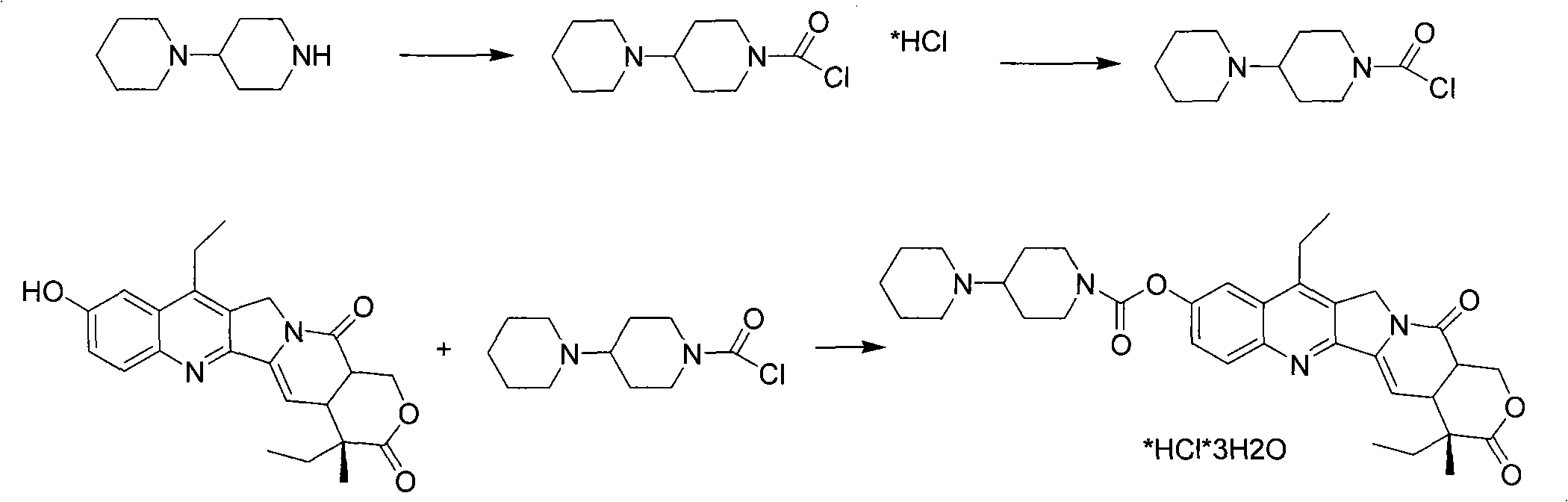
[0033] Prepare free 4-piperidinyl piperidine formyl chloride alkali: dissolve 2.2Kg of 4-piperidinyl piperidine formyl chloride hydrochloride in 10Kg of dichloromethane, add 1Kg of sodium bicarbonate and 5Kg of aqueous solution, and After stirring and reacting for 1 hour, the organic phase was separated and concentrated to remove the solvent, and 28 Kg of pyridine was added to dissolve it for later use.
[0034] Feeding reaction: put 20L pyridine into a clean 100L reactor, start the stirring device, stir fully, add 2Kg of SN-38 refined product, stir at room temperature for 1 hour, after it is completely dissolved, add the above-mentioned 4-piperidinylpiperidine methyl dropwise acid chloride solution. Add about 3 hours. After dropping, react at 25-30°C for 10 hours.
[0035] Post-processing: heating to 50°C, and distilling off pyridine under reduced pressure. After cooling to 40°C, add 50L of chloroform, fu...
Embodiment 4
[0036] Embodiment 4 salt refining
[0037] Feeding reaction: Add 35L deionized water to a 100L dissolution kettle, add 2Kg crude irinotecan, 0.7L concentrated hydrochloric acid, start the stirring device, heat to 50°C, and dissolve completely.
[0038] Post-treatment: Cool to 30°C, add 20L chloroform, stir well, separate layers, decolorize with activated carbon. Filter and concentrate the filtrate to 25L of water, cool and crystallize at 5°C, filter and wash with cold water. After drying, 2.1 Kg of white crystalline solid was obtained. The molar yield is 89%. HPLC analysis showed that the purity of the product was >99%, and the purity of the product was less than 0.1%.
[0039] In summary, the production method of irinotecan of the present invention eliminates the need for column chromatography in the refining process of irinotecan, shortens the production cycle, simplifies operations, reduces costs, increases yield, and is suitable for industrial scale production.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com