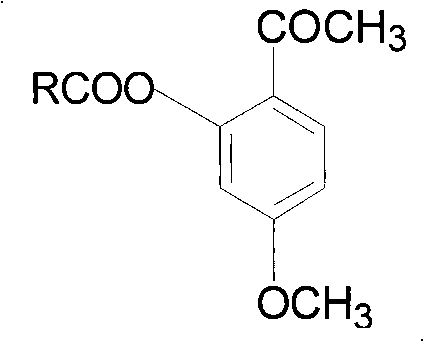
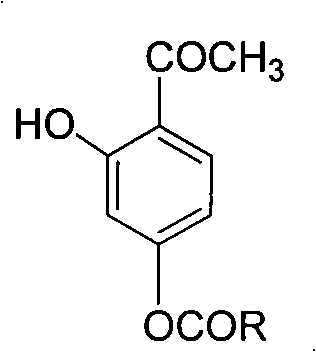
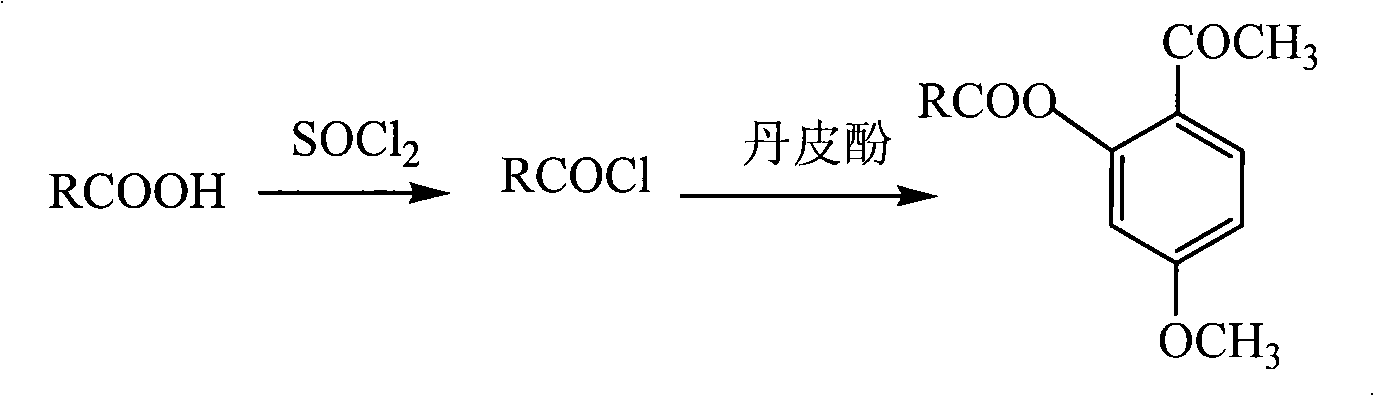
Method for preparing novel nonsteroidal anti-inflammatory drug and anti-inflammatory and analgesic effects thereof
A technology for non-steroidal anti-inflammatory drugs and compounds, which can be used in anti-inflammatory agents, anti-infective drugs, pharmaceutical formulations, etc., and can solve the problems of unstable room temperature storage, easy volatility, poor water solubility and low melting point.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: Synthesis of the synthesis of 5-methoxy-2-acetylphenyl ibuprofen ester (AXC 1 )
[0024] In a 25mL three-necked flask, add ibuprofen (1.03g, 5.0mmol) and thionyl chloride (1.5mL, 20.0mmol), reflux for 2h, and distill off the thionyl chloride under reduced pressure. The residue was dissolved in 10 mL of anhydrous dichloromethane, cooled to 0°C in an ice bath, paeonol (0.83 g, 5.0 mmol) and pyridine (0.8 mL, 10.0 mmol) were added, stirred for 2 h under ice bath, and then 10 mL of water was added , dichloromethane extraction (10mL × 3), combined organic phase, successively with saturated NaHCO 3 solution, distilled water and saturated saline solution, anhydrous MgSO 4 dry. After filtration, the filtrate was concentrated and subjected to column chromatography, and the eluent was petroleum ether:ethyl acetate (v:v)=6:1. The eluents were combined, the solvent was evaporated, and the oil pump was pumped to dryness to obtain a light yellow solid with a yield o...
Embodiment 2
[0025] Embodiment 2: the synthesis (AXC 2 )
[0026] In a 25ml round bottom flask, add ketoprofen (1.0g, 4.0mmol), thionyl chloride (1.2mL, 16.0mmol), pyridine 1.0mL, and reflux for 1h. Evaporate excess thionyl chloride under reduced pressure, dissolve the residue in 10 mL of anhydrous dichloromethane, cool in an ice bath, add paeonol (0.60 g, 3.0 mmol) and triethylamine (1.4 mL, 10.0 mL), Stir at room temperature for 3h. Add 0.5N sodium hydroxide solution, continue to stir for 20min, extract with dichloromethane (15mL×3), combine the organic phases, anhydrous MgSO 4 dry. After filtration, the filtrate was concentrated and subjected to column chromatography, and the eluent was petroleum ether:ethyl acetate (v:v)=9:1. The eluents were combined, and the solvent was evaporated to obtain an oily product with a yield of 68.2%. IR (cm -1 , KBr): 1759.2 (C=O), 1643.5 (C=O); 1 HNMR (400MHz, CDCl 3 , TMS), δ: 1.71 (d, 3H, CH 3 ), 2.42 (s, 3H, CH 3 ), 3.80 (s, 3H, CH 3), 4.14...
Embodiment 3
[0027] Embodiment 3: 3-hydroxyl-4-acetylphenyl ibuprofen ester (AXC 3 )
[0028] In a 25mL round bottom flask, 2,4-dihydroxyacetophenone (0.76g, 5.0mmol) was dissolved in 8mL of anhydrous dichloromethane, then added ibuprofen (1.03g, 5.0mmol), DCC (1.03g , 5.0mmol) and several grains of DMAP were stirred until dissolved. After 2h, filter, concentrate the filtrate, and perform column chromatography. The eluent is petroleum ether:ethyl acetate (v:v)=9:1. The eluents are combined, the solvent is evaporated, and the oil pump is drained to obtain an oily substance. Yield 71.25%. IR (cm -1 , KBr): 3500~3100 (OH), 1766.9 (C=O), 1643.5 (C=O); 1 HNMR (400MHz, CDCl 3 , TMS), δ: 0.80 (d, 6H, CH 3 ), 1.60 (d, 3H, CH 3 ), 1.90(m, 1H, CH), 2.55(d, 2H, CH 2 ), 2.72 (s, 3H, CH 3 ), 3.80(m, 1H, CH), 6.59(m, 2H, ArH), 7.16(d, 2H, J=7.2Hz, ArH), 7.27(d, 2H, J=7.6Hz, ArH), 7.80( d, 1H, J=8.0Hz, ArH), 12.40(s, 1H, OH); HR-MS: m / z Calcd for C 21 h 24 o 4 340.1675, Found: 340.1651 (M +...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com