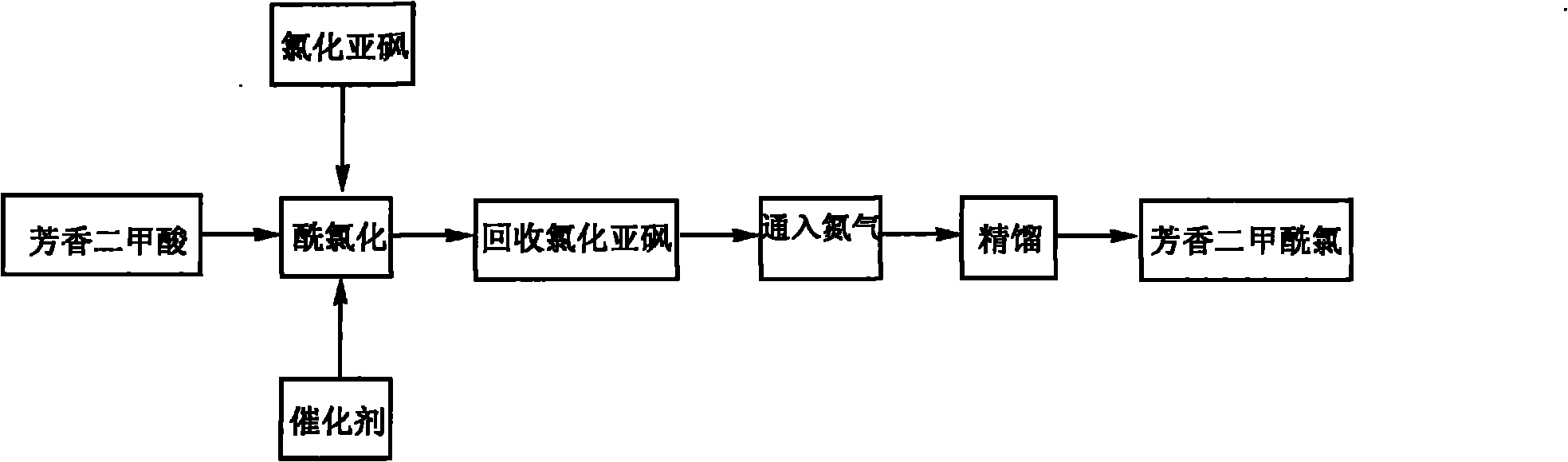
Method for preparing aromatic dimethyl chloride
A diacid chloride and aromatic technology, which is applied in the field of preparation of aromatic diacid chloride, can solve the problems of many by-products, low purity, and low product purity, and achieve the effect of increasing yield and improving purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Embodiment 1, preparation isophthaloyl dichloride
[0013] Add 3300Kg of thionyl chloride and 12Kg of pyridine into the batching tank, stir and mix, and after mixing, add them into the synthesis kettle together with 1500Kg of isophthalic acid. Turn on the vacuum pump, reflux condenser and tail gas absorption device, control the vacuum degree to 40mmHg, and react at 80°C for 20 hours. After the reaction is completed, increase the vacuum degree to be greater than or equal to 0.09MPa, control the temperature in the kettle to 115°C, and recover the thionyl chloride by evaporation. Feed nitrogen into the kettle, keep the vacuum degree greater than or equal to 0.09MPa, control the temperature in the kettle to 125°C, and exhaust the waste gas in the kettle. Cool the reaction solution to 90°C and transfer it to the still, raise the temperature of the still to 120-130°C under a vacuum of 0.09MPa, collect about 130Kg of the fore distillation, and collect in the range of 130-140°...
Embodiment 2
[0015] Embodiment 2, preparation terephthaloyl chloride
[0016] Add 3250Kg of thionyl chloride and 11.5Kg of N-methylpyrrolidone into the batching tank, stir and mix, and after mixing, add them into the synthesis kettle together with 1500Kg of terephthalic acid. Turn on the vacuum pump, reflux condenser and tail gas absorption device, control the vacuum degree to 40mmHg, and react at 110°C for 20 hours. After the reaction is completed, increase the vacuum degree to be greater than or equal to 0.09MPa, control the temperature in the kettle to 125°C, and recover the thionyl chloride by evaporation. Feed nitrogen into the kettle, keep the vacuum degree greater than or equal to 0.09MPa, control the temperature in the kettle to 135°C, and exhaust the waste gas in the kettle. The reaction solution was cooled to 90°C and transferred to a still. At a vacuum of 0.09MPa, raise the temperature of the still to 110-120°C, collect about 110Kg of the fore distillation, and collect 1770Kg ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com