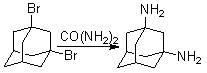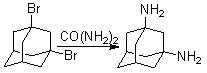Method for synthesizing 1, 3-adamantane diamine
A technology of adamantanediamine and its synthesis method, which is applied in the preparation of amino-substituting functional groups, organic chemistry, etc., can solve the problems of expensive raw materials and catalysts, high risk of reagents, etc., and achieve mild conditions, easy-to-obtain raw materials, and high-efficiency products. high effect
Inactive Publication Date: 2011-06-15
GUANGDONG UNIV OF TECH
View PDF3 Cites 4 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The yield of this method is relatively high, but the reagents used are highly dangerous, and the raw materials and catalysts are expensive.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention discloses a method for synthesizing 1, 3-adamantane diamine. The 1, 3-adamantane diamine synthesized by the method is prepared by the following steps of: performing amination on 1, 3-dibromoadamantane and urea in a high boiling point solvent; and performing separated purification through acidification, neutralization and extraction. The method particularly comprises the following steps of: mixing the 1,3-dibromoadamantane and the urea uniformly according to the molar ratio of the 1,3-dibromoadamantane to the urea of 1:2-10; adding the high boiling point solvent; heating the mixture to a preset temperature in an oil bath and reacting under stirring; after the reaction is finished, cooling to 50 DEG C; adding hydrochloric acid and stirring to dissolve the product; filtering to remove insoluble impurities; adding sodium hydroxide solution into filtrate to neutralize; adding an excessive organic solvent to perform extraction; distilling the obtained extract liquid under reduced pressure to remove the solvent; and performing vacuum drying to obtain the product 1,3-adamantane diamine. The method has the advantages of short reaction route, simpleness and convenience in operation, mild condition, clean and environmentally-friendly process, high product yield and the like.
Description
A kind of synthetic method of 1,3-adamantanediamine technical field The invention relates to a synthesis method of 1,3-adamantanediamine. Adamantanediamine is currently mostly used in polymer monomers, and polymers containing 1,3-adamantanediamine can be used in microelectronics, gas separation, etc. have potential medical value. Background technique 1,3-Adamantanediamine has important applications in the fields of polymer materials, fine chemicals, electronics, and medicine because it contains both a rigid adamantane cage structure and a highly active amine group. In the field of synthetic polymer materials, 1,3-adamantanediamine can be used to synthesize polyimide-based gas separation membrane materials with excellent performance, and can be used to synthesize organic light-emitting materials with high thermal stability, and can also be used as a polymerization monomer Or modifying groups are introduced into other polymer materials to change their thermal stability and...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07C211/38C07C209/08
Inventor 郭建维朱华钟星付长安蔡璐马倩彭进平崔亦华邓志城
Owner GUANGDONG UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com