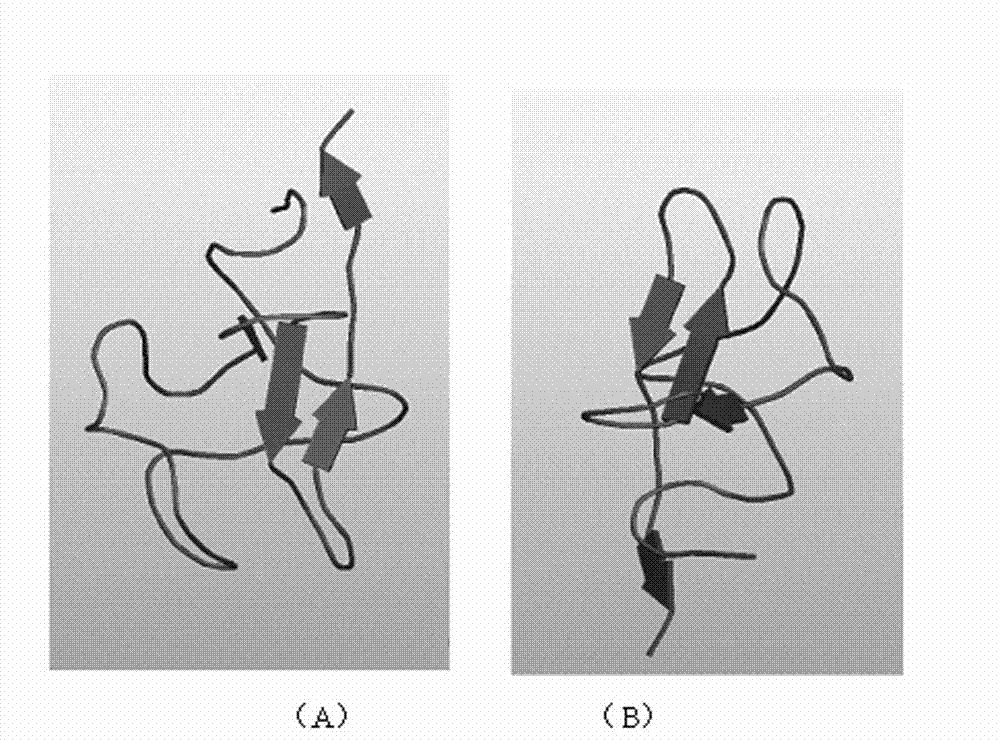
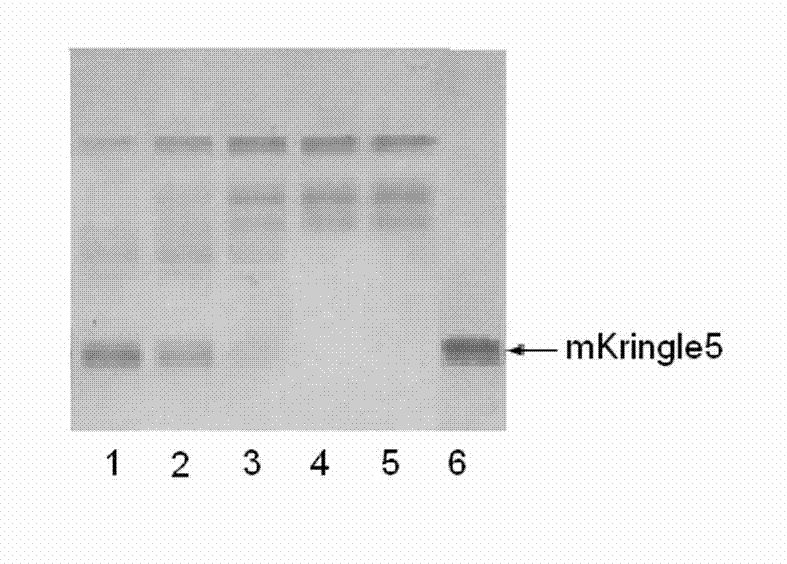
Kringle 5 mutant protein, and preparation method and application thereof
A mutant and protein technology, applied in the fields of molecular biology and medicine, can solve the problems of weak anti-angiogenic effect, difficult preparation cost, systemic toxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Acquisition of Kringle5 mutant protein coding gene (N sequence does not exist but C sequence exists, in vitro synthesis + PCR method).
[0027] If the C sequence is His-His-His-His-His-His, the following gene fragments can be designed and synthesized by Shanghai Sangong Company:
[0028] SEQ ID NO: 1 (76 bp): GCGAATTCCATGGACTGTACTGGGACGCCATGCCAGGACTGGGCTGCCCAGGAGCCCCATAGACACAGCATTTTCA
[0029] SEQ ID NO: 2 (78 bp): CCACCTACATCACCATCAGGGTTACGGCAGTAATTTTTTTTC CAGACCCGCCCGTGGATTTGTCTCTGGAGTGAAAATG
[0030] SEQ ID NO: 3 (73 bp): TGTAGGTGGTCCCTGGTGCTACACGACAAATCCAAGAAAAC TTTACGAC TACTGTGATGTCCCTCAGTGTGCG
[0031] SEQ ID NO: 4 (49 bp): GTCCCTCAGTGTGCCCACCACCACCACCACTAATAGCGC ACACTGA
[0032] Restriction sites, start codons, and stop codons were introduced into the above-mentioned gene fragments, and the 5' of the synthesized nucleic acid fragments were phosphorylated, routinely extracted with phenol and chloroform, and conventional PCR reaction operations were performed. Th...
Embodiment 2
[0035] Acquisition of mKringle5 protein coding gene (both N sequence and C sequence exist, in vitro synthesis + PCR method).
[0036] If the N sequence is Phe-Glu, and if the C sequence is His-His-His-His-His-His, the following gene fragments can be designed and synthesized by Shanghai Sangon:
[0037] SEQ ID NO: 6 (82 bp): GCGAATTCCATGTTTGAAGACTGTACTGGGACGCCATGCC AGGACTGGGCTGCCCAGGAGCCCCATAGACACAGCATTTTCA
[0038] SEQ ID NO: 6 (78 bp): CCACCTACATCACCATCAGGGTTACGGCAGTAATTTTTTTTC CAGACCCGCCCGTGGATTTGTCTCTGGAGTGAAAATG
[0039] SEQ ID NO: 8 (73 bp): TGTAGGTGGTCCCTGGTGCTACACGACAAATCCAAGAAAAC TTTACGAC TACTGTGATGTCCCTCAGTGTGCG
[0040] SEQ ID NO: 9 (49 bp): GTCCCTCAGTGTGCCCACCACCACCACCACTAATAGCG CACACTGA
[0041] A restriction site, a His-tag tag, a start codon, and a stop codon were introduced into the above gene fragment. Phosphorylate the 5' of the synthesized nucleic acid fragments, and routinely perform phenol and chloroform extraction. Carry out routine PCR reaction operat...
Embodiment 3
[0043] The 5′ and 3′ ends of the mKringle5 protein gene (abbreviated as mKringle5 gene) were cut with restriction endonucleases, and the empty vector (such as pBV220) planned to be used was also cut with the same enzyme, and then ligated in vitro ( That is, recombination), the vector containing the gene sequence of the Kringle5 mutant protein can be obtained. Next, the preparation of the pBV220 / mKringle5 mutant protein recombinant plasmid expression vector will be described in detail.
[0044] PCR primer design: According to the gene sequence of Kringle5 mutant protein, the following primers can be designed: primer 1: 5′-GCGAATTCCATGGACTGTACTGGGACG–3′, primer 2: 5′-GCGGATCCCCTATTAGTGG TGGTGGT GGTGGTGGGCACACTGAGGGAC–3′. Among them, GAATTC and GGAT CC are restriction endonucleases EcoRI and BamHI restriction sites; ATG is a start codon; TAATAG is a stop codon; GTGGTGGTGGT GGTGGTG is a sequence of 6 histidines; 5′GC is a protection sequence .
[0045] PCR amplification of Kring...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com