Variable-conformation recombinant interferon crystal, and three-dimensional structure and use thereof
A technology of recombinant interferon and three-dimensional structure, applied in the field of recombinant interferon crystals, can solve the problems of spatial conformation and biological efficacy changes, and achieve the effects of low side effects, high antiviral activity, and reduced toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] Preparation of recombinant interferon rSIFN-co
[0117] This embodiment is a preparation method of recombinant interferon rSIFN-co (hereinafter also referred to as "rSIFN-co") (stock solution) (for details, refer to US Patent No. 7,364,724 Specifications Examples 1 and 2 and Chinese Patent Publication No. method described on pages 11-17).
[0119]According to the published coding DNA sequence and deduced amino acid sequence data (Klein ML, Bartley TD, Lai PH, et al., Structural characterization of recombinant consensus interferon-alpha. Journal of Chromatography, 1988; 454: 205-215), the large intestine Bacillus expresses codons preferentially (The Wisconsin Package, by Genetics Computer Group, Inc. Copyright 1992, Medison, Wisconsin, USA). Under the condition that the amino acid sequence remains unchanged, molecular design is carried out on its DNA coding sequence, and then artificially synthesized. rSIFN-co full-length cDNA encoding gene....
Embodiment 2
[0188] Recombinant Interferon Preparations
[0189] Formula for freeze-dried injection (lyophilized powder)
[0190] rSIFN-co stock solution of the present invention 34.5 μg / ml
[0191] Phosphate buffer at pH 7.0 10mmol / L
[0192] Glycine 0.4mol / L
[0193] Preparation process: weigh according to the formula, dissolve in sterile pyrogen-free injection water, filter and sterilize through a 0.22 μm pore-diameter filter membrane, store at 6-10°C, take samples for sterility and pyrogen inspection, and then pack them into vials. A single dose of 0.3-0.5 vials is divided and placed in a lyophilizer for freeze-drying.
[0194] Formulation of aqueous solution injection
[0195] rSIFN-co stock solution of the present invention 34.5 μg / ml
[0196] Phosphate buffer at pH 7.0 25mmol / L
[0197] Sodium chloride 0.4mol / L
[0198] Preparation process: Weigh according to the formula, dissolve in sterile pyrogen-free injection water, filter and sterilize through a 0.22μm pore-diameter f...
Embodiment 3
[0200] Crystallization of recombinant interferon
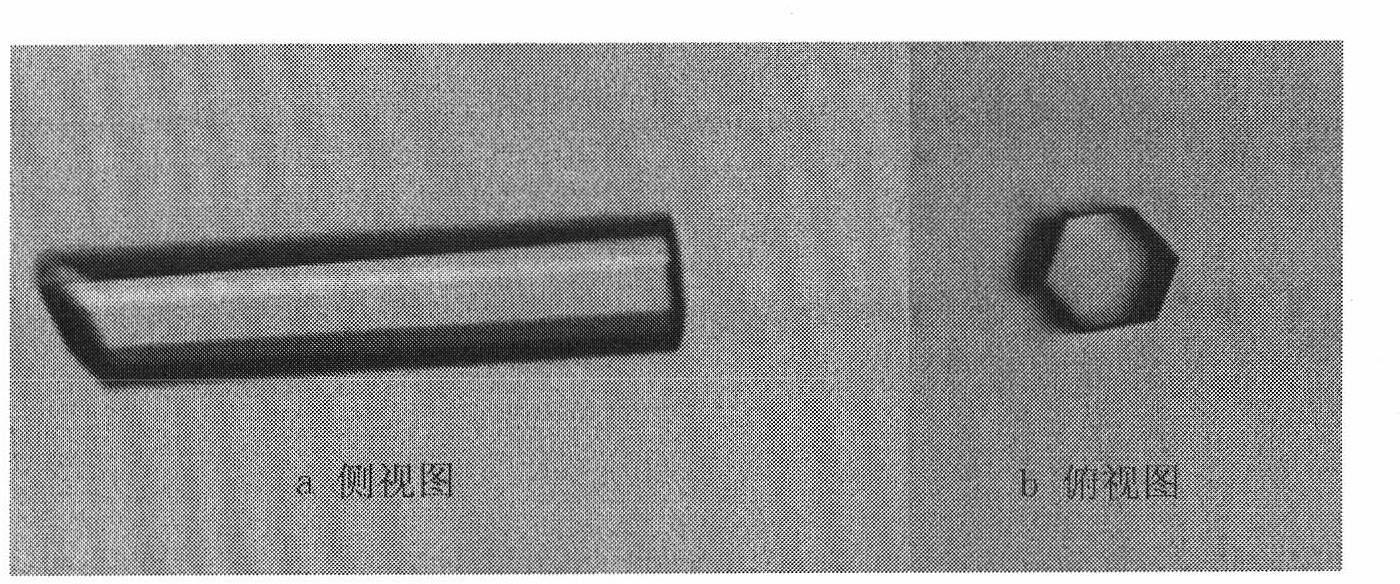
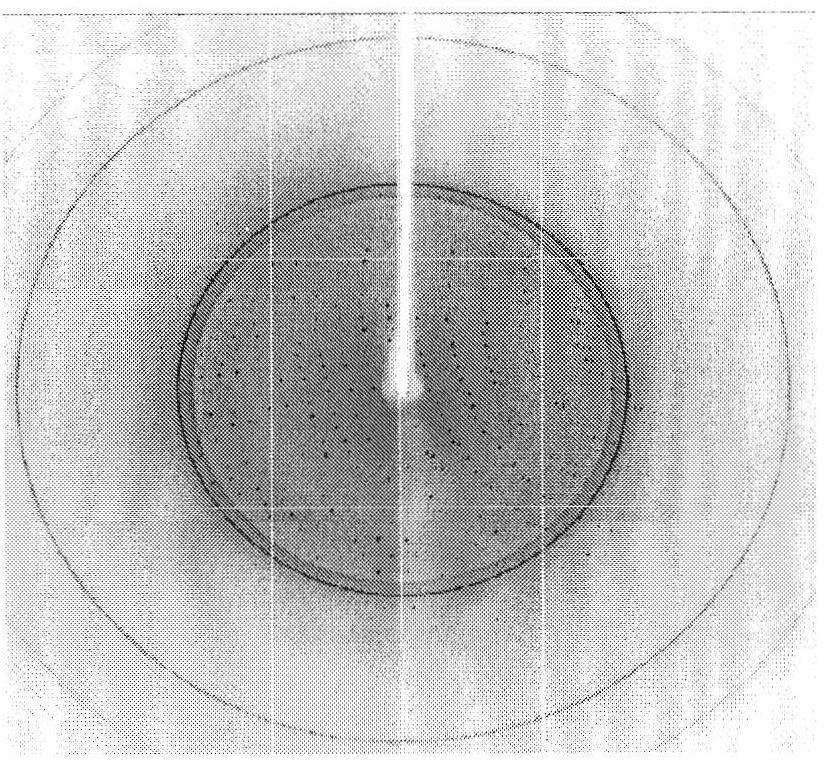
[0201] Preparation of high-quality rSIFN-co protein single crystal is a prerequisite for determination of its crystal structure. The rSIFN-co samples used for crystal growth were obtained from the rSIFN-co of the present invention as described above. The single crystal preparation method, technical process and crystallization conditions of rSIFN-co, and its crystallographic parameters are as follows:
[0202] The above-mentioned rSIFN-co freeze-dried powder of the present invention was dissolved in pure water and stored at low temperature (-20° C.), with an initial protein concentration of 0.42 mg / ml. The rSIFN-co protein samples were concentrated to 3-3.5 mg / ml before crystallization and immediately used for crystal growth experiments. Crystal cultivation was carried out at room temperature (293K) by the hanging drop vapor phase diffusion method.
[0203] In the early stage of crystallization research, microcrystals of r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com