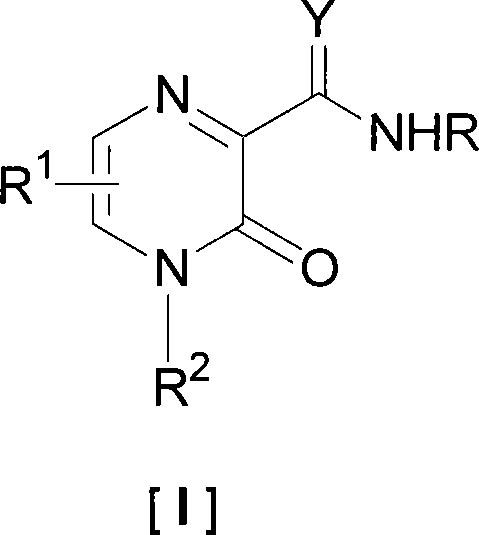
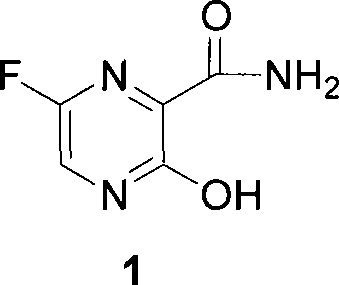
3-oxide-2-methylamide derivatives as well as preparation method and use thereof
A technology of oxypyrazine and formamide, which is applied in the field of pyrazine-2-carboxamide derivatives, can solve the problems of low water solubility, achieve the effects of improved solubility, improved antiviral activity, and high yield of finished products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0030] Synthesis of compound 9:
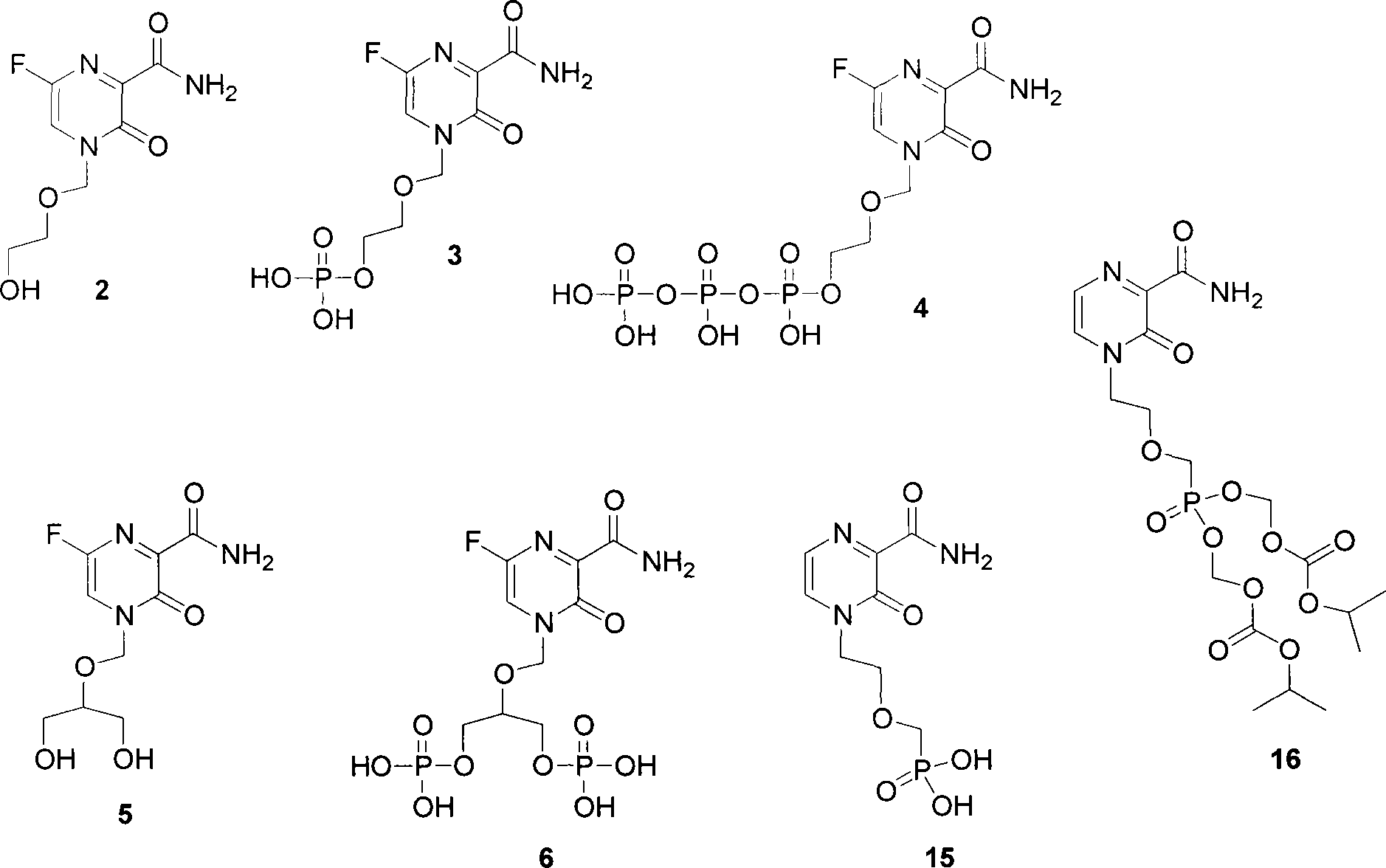
[0031] Compound 7 (1.72 g, 0.01 mol) was dissolved in 30 ml of DMF, and K 2 CO 3 (3g), 2-chloromethoxyethyl acetate 8 (1.53g, 0.01mol). A small amount of KI was added to the mixture, and stirred at room temperature for 12 hours. The solvent was evaporated under reduced pressure, and the residue was dissolved in 100 ml of chloroform, Washed once with water, washed with brine, and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure, and the residue was separated by silica gel column chromatography (using 10% methanol-dichloromethane) to obtain compound 9 (1.95 g, yield 68%). The melting point is 188-190°C. HRMS (ESI) Calcd.for C 11 h 13 FN 2 o 6 : 288.2292; Found 288.2290.
Synthetic example 2
[0033] Synthesis of compound 2:
[0034] Compound 9 (1 g, 0.0035 mol) and 7N methanol solution of ammonia were mixed and sealed at room temperature for three days. The solvent was evaporated under reduced pressure, and the residue was separated by silica gel column chromatography (using 10% methanol-dichloromethane) to obtain Compound 2 (0.75 g, 93%). Melting point 212-215°C. HRMS (ESI) Calcd.for C 8 h 10 FN 3 o 4 : 231.1812; Found 231.1810.
Synthetic example 3
[0036] Synthesis of Compound 10:
[0037] Compound 2 (2.31 g, 0.01 mol) was dissolved in 70 mL of anhydrous dichloromethane, tetra-tert-butoxy (0.4 mL, 0.01 mol), triethylamine (2.12 mL, 0.015 mol) and diethyl chlorophosphate were added (1.42 mL, 0.01 mol). The mixture was stirred at room temperature for 24 hours, 80 ml of water was added, the organic layer was separated, and the aqueous phase was extracted with ethyl acetate (3×100 mL). The organic phases were combined, dried over anhydrous sodium sulfate, concentrated in vacuo, and the residue was subjected to silica gel column chromatography Compound 10 was isolated (5% methanol-dichloromethane) (3.2 g, 87% yield). HRMS (ESI) Calcd.for C 12 h 19 FN 3 o 7 P: 368.2674; Found 368.2675.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com