Amantadine hydrochloride and resveratrol pharmaceutical co-crystal and preparation method thereof
A technology of amantadine hydrochloride and resveratrol, which is applied in the field of drug co-crystals, can solve the problems of toxic side effects and drug efficacy reduction, and achieve the effects of reducing toxic side effects, high yield, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: The preparation method of amantadine hydrochloride and resveratrol drug co-crystal is implemented according to the following steps:
[0030] Add amantadine hydrochloride (56.31 mg) and resveratrol (34.24 mg) in a molar ratio of 2:1 and mix them in a mortar, add 50 mL of isopropanol and quickly grind until dry, and continue grinding for 1 h to obtain a white powder , transfer the white powder to a round bottom flask, add 17 mL of acetonitrile and 1.7 mL of methanol (volume ratio: 10:1) mixed solvent to dissolve, stir for 4 h and then filter, leave the filtrate to evaporate for 3 days to obtain colorless columnar crystals.
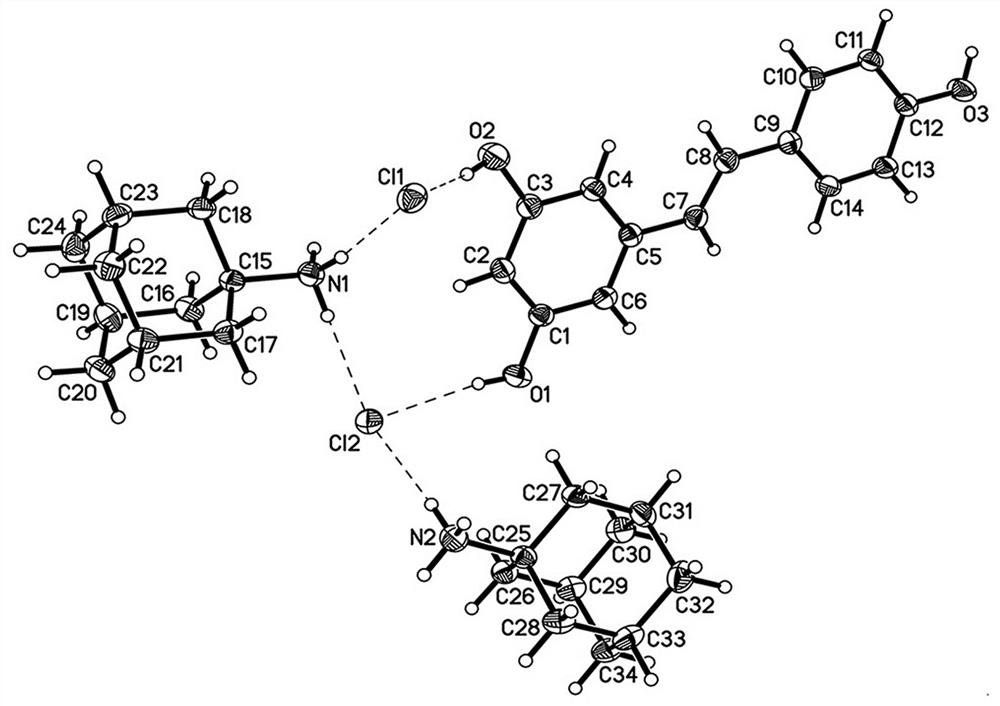
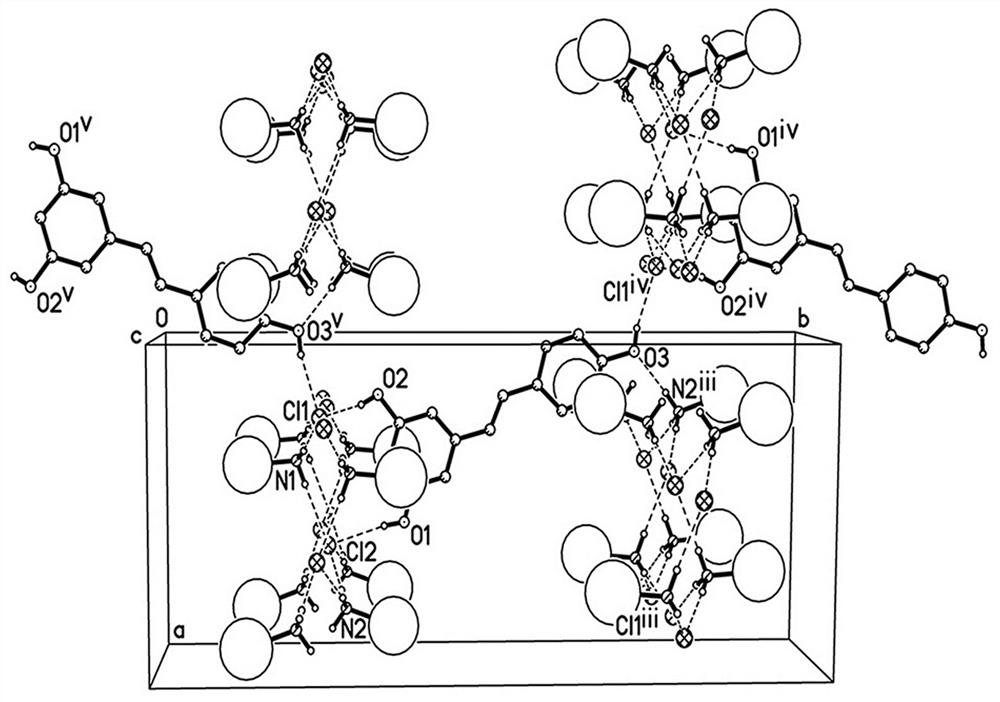
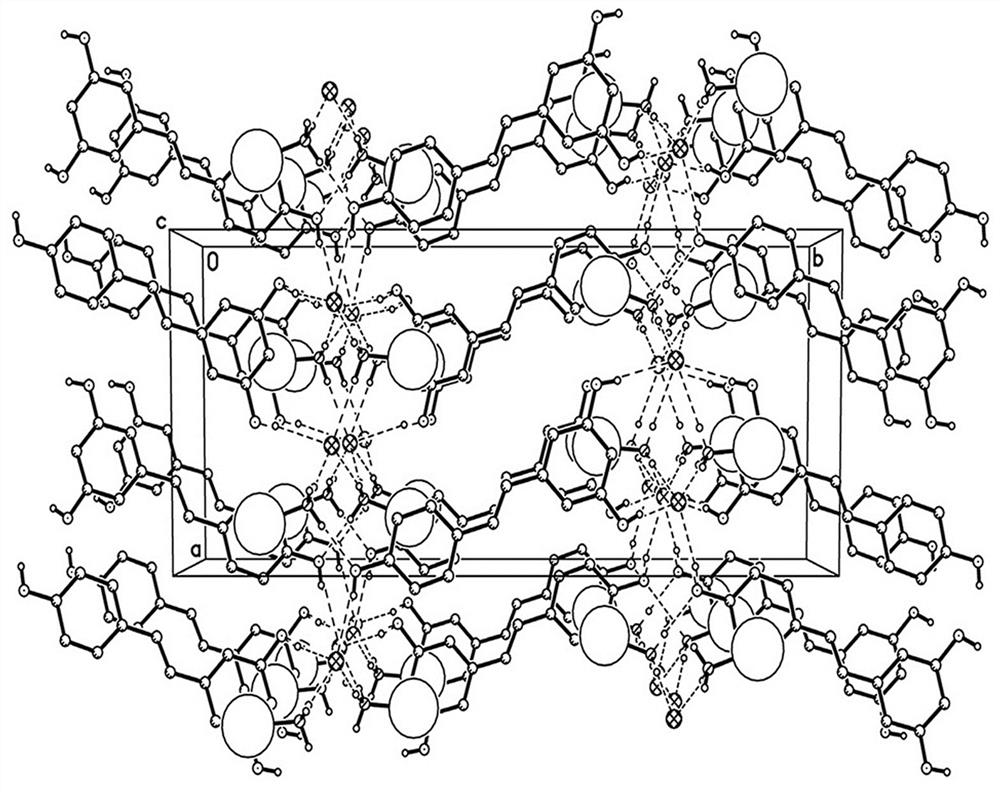
[0031] In this example, the drug co-crystal of amantadine hydrochloride and resveratrol prepared by the solvent evaporation method is a colorless columnar crystal, and a single crystal sample with an appropriate size is selected for X-ray single crystal diffraction measurement. The STADIVARI diffractometer of STOE Company in Germany is u...
Embodiment 2
[0035] Embodiment 2: The preparation method of amantadine hydrochloride and resveratrol drug co-crystal is implemented according to the following steps:
[0036] The difference between this embodiment and Example 1 is that the volume ratio of acetonitrile and methanol in the system is 7:1, and other steps and parameters are the same as in Embodiment 1.
Embodiment 3
[0037] Embodiment 3: The preparation method of amantadine hydrochloride and resveratrol pharmaceutical co-crystal is implemented according to the following steps:
[0038] The difference between this embodiment and Example 1 is that the volume ratio of acetonitrile and methanol in the system is 13:1, and other steps and parameters are the same as in Embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com