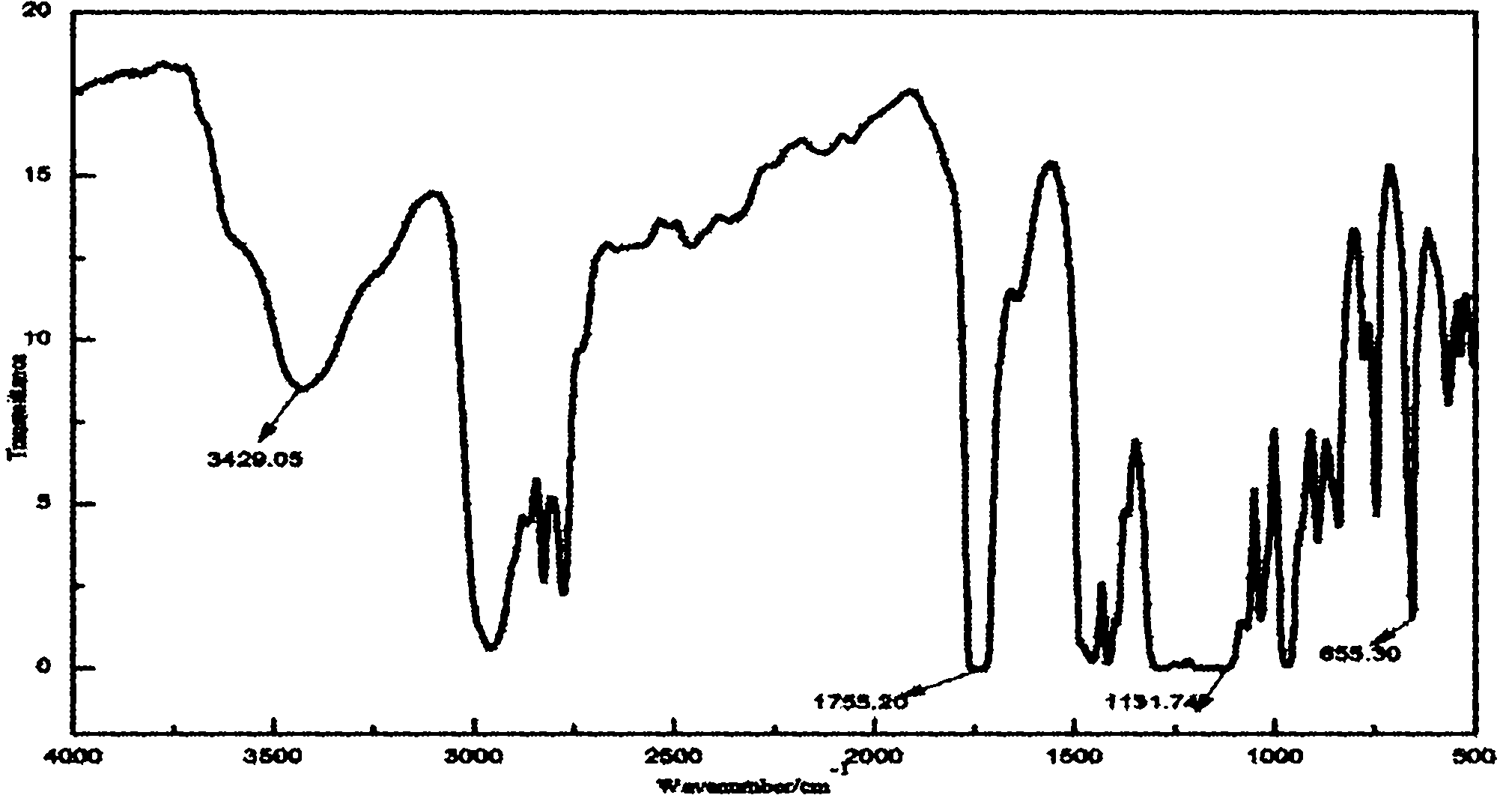
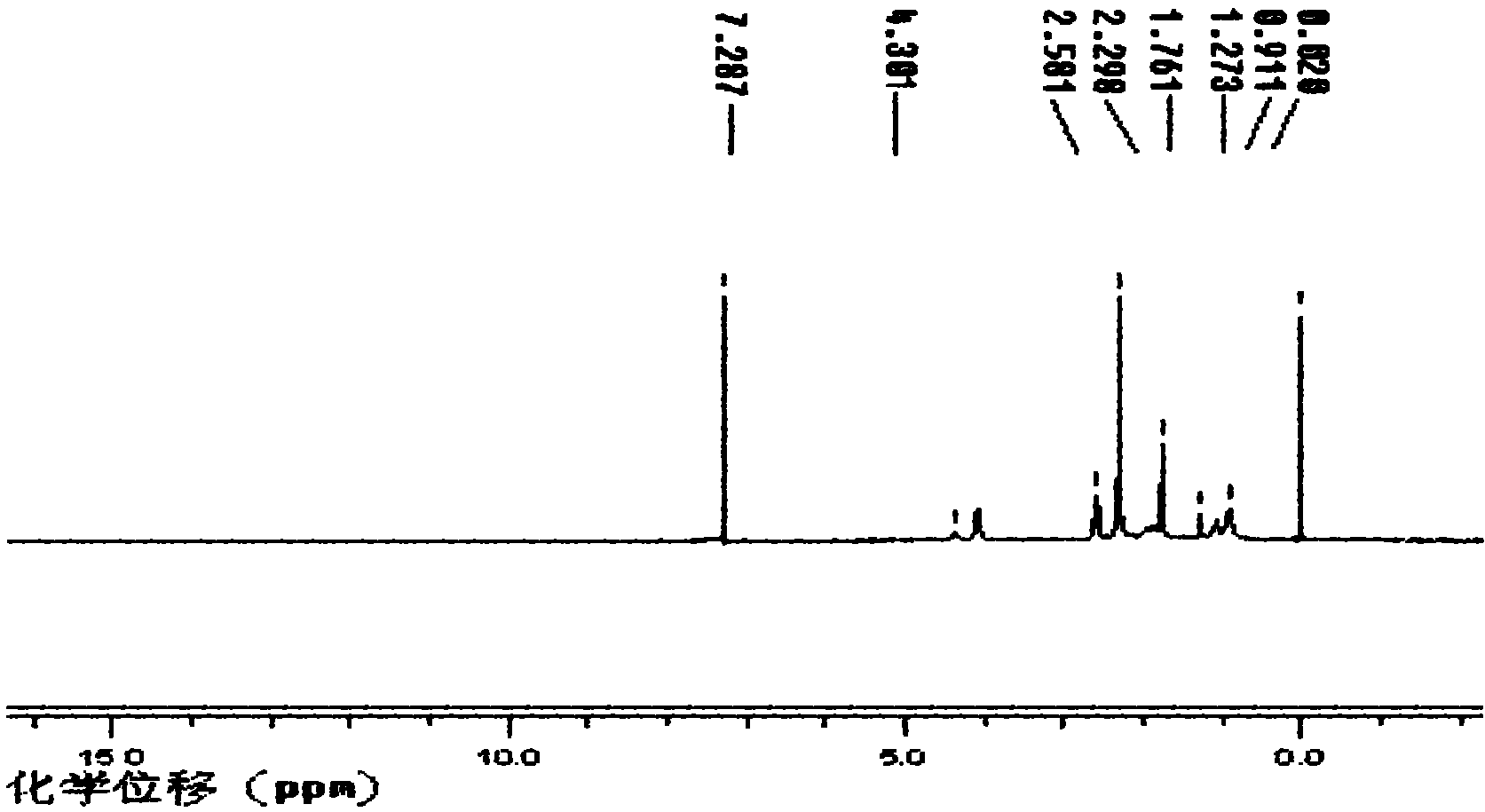
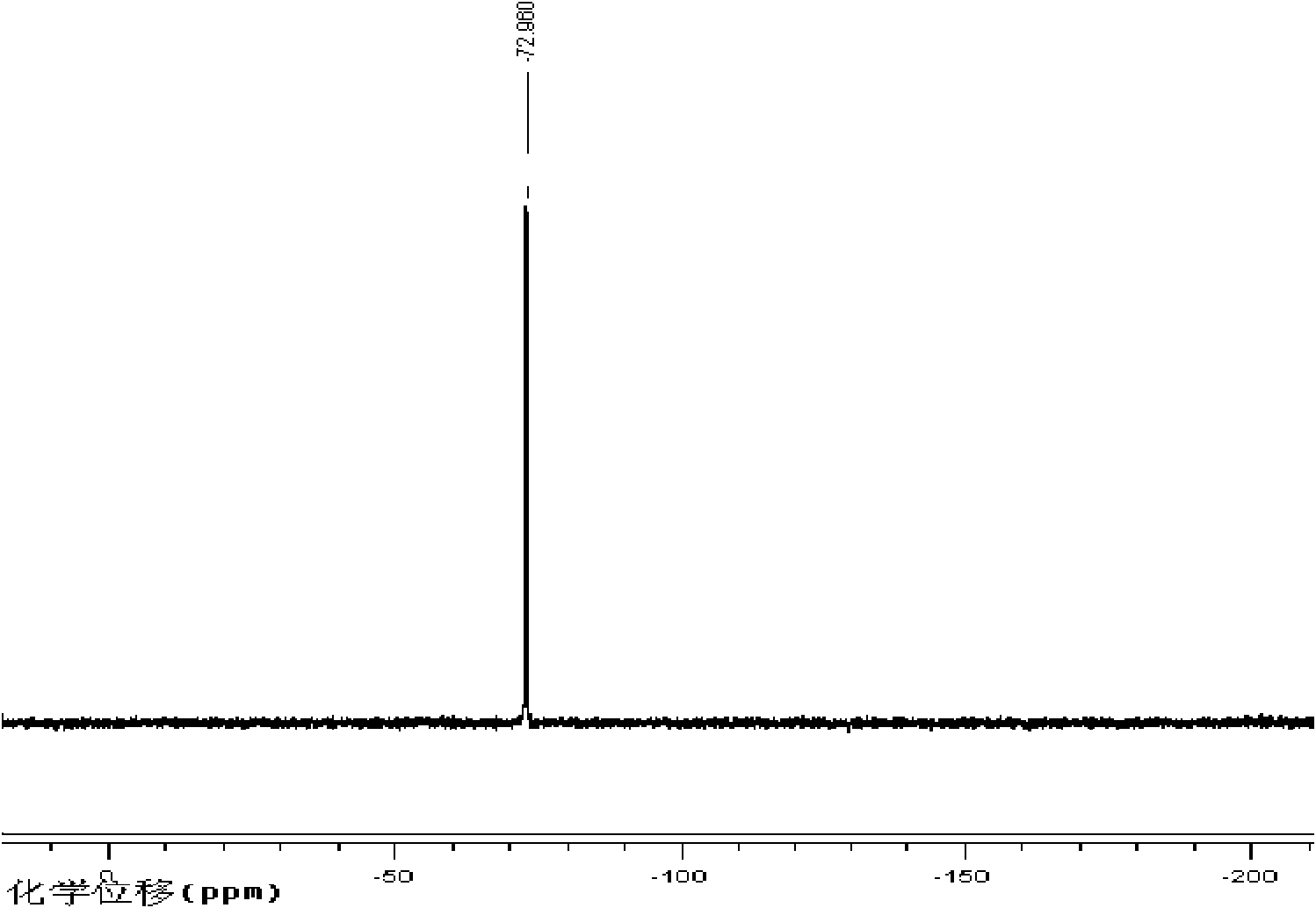
Fluorine-containing acrylate polymer anion exchange membrane and preparation method thereof
An anion exchange membrane and acrylate technology, applied in the field of ion exchange membrane, can solve the problems of thermal stability, poor chemical stability, easy degradation, etc., and achieve the effect of inhibiting methanol permeation, good mechanical properties and chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Fluorinated Acrylate Polymer Anion Exchange Membrane
[0033] Add 0.01mol of dimethylaminoethyl methacrylate, 0.01mol of trifluoroethyl methacrylate, 10ml of tetrahydrofuran and 0.043g of azobisisobutyronitrile into a three-necked flask, and place at 65 ℃ water bath temperature, stirred and refluxed for 12h. The product was precipitated with ice water, washed with methanol to remove unreacted monomers, and dried at 65°C to obtain a polymer with tertiary amine groups.
[0034] 1 g of the above polymer was fully dissolved in 20 ml of N, N-dimethylformamide, vacuum degassed, cast on a clean glass plate to form a film, and dried at 60°C for 24 hours. The cast membrane was then placed in a 1mol L -1 In aqueous hydrochloric acid solution, after reacting at 30°C for 24 hours, rinse with a large amount of water until there is no hydrochloric acid, and place in 2mol·L -1 Transformation in NaOH solution to obtain hydrogen-oxygen type fluorine-containing acrylate polymer anion ...
Embodiment 2
[0041] The same method as in Example 1 was used, except that the amount of dimethylaminoethyl methacrylate was 0.02 mol, and the amount of trifluoroethyl methacrylate was 0.01 mol.
[0042] It was determined that the water content of the membrane was 19%, and the ion exchange capacity was 2.916mmol·g -1 . The methanol permeability coefficient of the membrane is less than 10 at room temperature -8 cm 2 ·s -1 ,Such as Figure 5 As shown in the middle curve C, the methanol permeability coefficient of the membrane increases with the increase of temperature. The conductivity of the film at room temperature is 0.05 S cm -1 , the conductivity varies with temperature as Figure 6 As shown in the middle curve C, the conductivity of the film increases with the increase of temperature, and it can still maintain a high conductivity at a high temperature of 95 °C.
Embodiment 3
[0044] The same method as in Example was used, except that the amount of dimethylaminoethyl methacrylate was 0.05 mol, and the amount of trifluoroethyl methacrylate was 0.1 mol.
[0045] It was determined that the water content of the membrane was 33.2%, and the ion exchange capacity was 1.197mmol·g -1 . The methanol permeability coefficient of the membrane is less than 10 at room temperature -8 cm 2 ·s -1 ,Such as Figure 5 As shown in the middle curve A, the methanol permeability coefficient of the membrane increases with the increase of temperature. The conductivity of the film at room temperature is 0.01 S cm -1 , the conductivity varies with temperature as Figure 6 As shown in the middle curve A, the conductivity of the membrane increases with the increase of temperature, and it can still maintain a high conductivity at a high temperature of 95 °C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Methanol permeability coefficient | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com