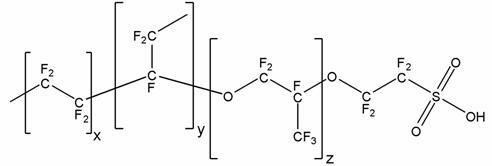
Process of producing 1,1 diaryl alkanes and derivatives thereof
A technology for diarylalkanes and aromatic compounds, applied in the field of manufacturing 1,1-diarylalkanes, can solve the problems of poor isomer selectivity, unsatisfactory reaction yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
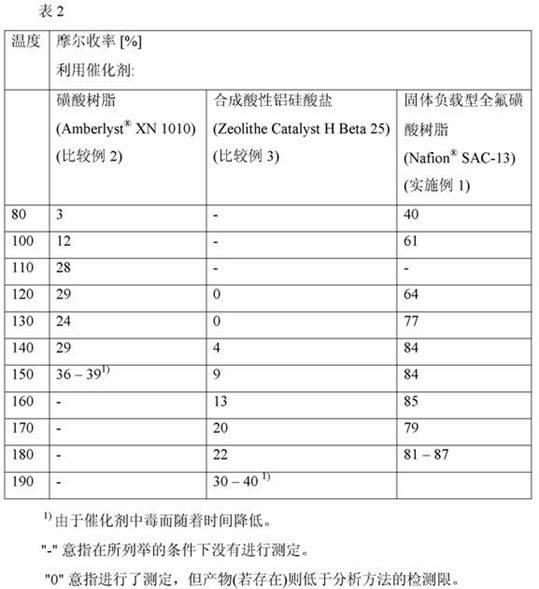
[0040] A tubular reactor (2.54 cm x 38.1 cm stainless steel) was filled with 18.01 grams of solid-supported perfluorosulfonic acid resin (Nafion® SAC-13). The catalyst bed was dried by heating to 150°C overnight under a nitrogen purge. The filled reactor was cooled to ambient temperature and a back pressure regulator set at 1379 kPa was attached to the outlet of the reactor. The system was primed with o-xylene at a flow rate of 0.89 mL / min and the reactor bed was heated to 170°C. Once the reactor bed was at temperature, the pure ortho-xylene feed was replaced with 6 wt% DMM in ortho-xylene and the flow rate was maintained at 0.89 mL / min. Analysis of the product stream leaving the tubular reactor showed that in 79 mol% yield, and with about 75% selection of the isomer 3,3',4,4'-tetramethyldiphenylmethane Proactively produces di(xylyl)methane (DXM).
Embodiment 2
[0042] The synthesis of DXM from DMM and o-xylene using solid-supported perfluorosulfonic acid resin (Nafion® SAC-13) was carried out using equipment similar to that described in Example 1.
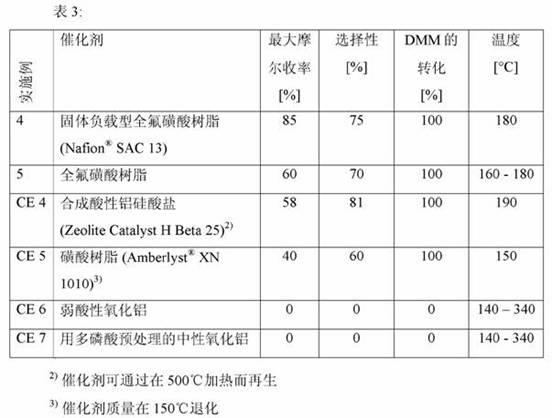
[0043]A catalyst-packed tube reactor was prepared by heating at 150° C. overnight under a nitrogen purge. The tube reactor was cooled to room temperature. Turn on the pump and establish a flow of the DMM / o-xylene mixture at 1.0 mL / min. The tube furnace temperature was set at 40°C and the reactor temperature was increased by 15°C every 0.5-2.0 hours. An increase in the molar yield of DXM was observed with temperature up to 140 °C. DMM was detected in the product stream until the reaction temperature reached 180°C. For airspeed 2.9hr -1 (1.0 mL / min) and 6 wt% DMM concentration, the best molar yield (85%), selectivity (70-75%) and DMM conversion (100%) occurred at 180 °C.
Embodiment 3
[0045] The synthesis of DXM from DMM and o-xylene using perfluorosulfonic acid resin was carried out using equipment similar to that described in Example 1.
[0046] A catalyst-packed tube reactor was prepared by heating at 150° C. overnight under a nitrogen purge. The tube reactor was cooled to room temperature. Turn on the pump and establish a flow of the DMM / o-xylene mixture at 1.0 mL / min. The tube furnace temperature was set at 40°C and the reactor temperature was increased until the reaction temperature reached 160°, 170° or 180°C, then held at this temperature for a period of 8-10 hours while periodically sampling the product stream. Analysis showed a constant DXM molar yield of 60%. It is also observed that the isoselectivity is sustained at 70% in this range.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com