Tanshinone compounds and applications thereof
A tanshinone and compound technology, applied to tanshinone compounds and their application fields, can solve the problem of no tanshinone compounds and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Preparation of Tanshinone Compounds
[0026] Take 200g of freshly dried plant Salvia miltiorrhiza, carry out continuous Soxhlet extraction with 80% ethanol, concentrate in vacuo to obtain ethanol extract, extract with ethyl acetate, carry out silica gel column chromatography, use petroleum ether, ethyl acetate and acetic acid Gradient elution was carried out to obtain tanshinone I, tanshinone IIA, cryptotanshinone and dihydrotanshinone.
Embodiment 2
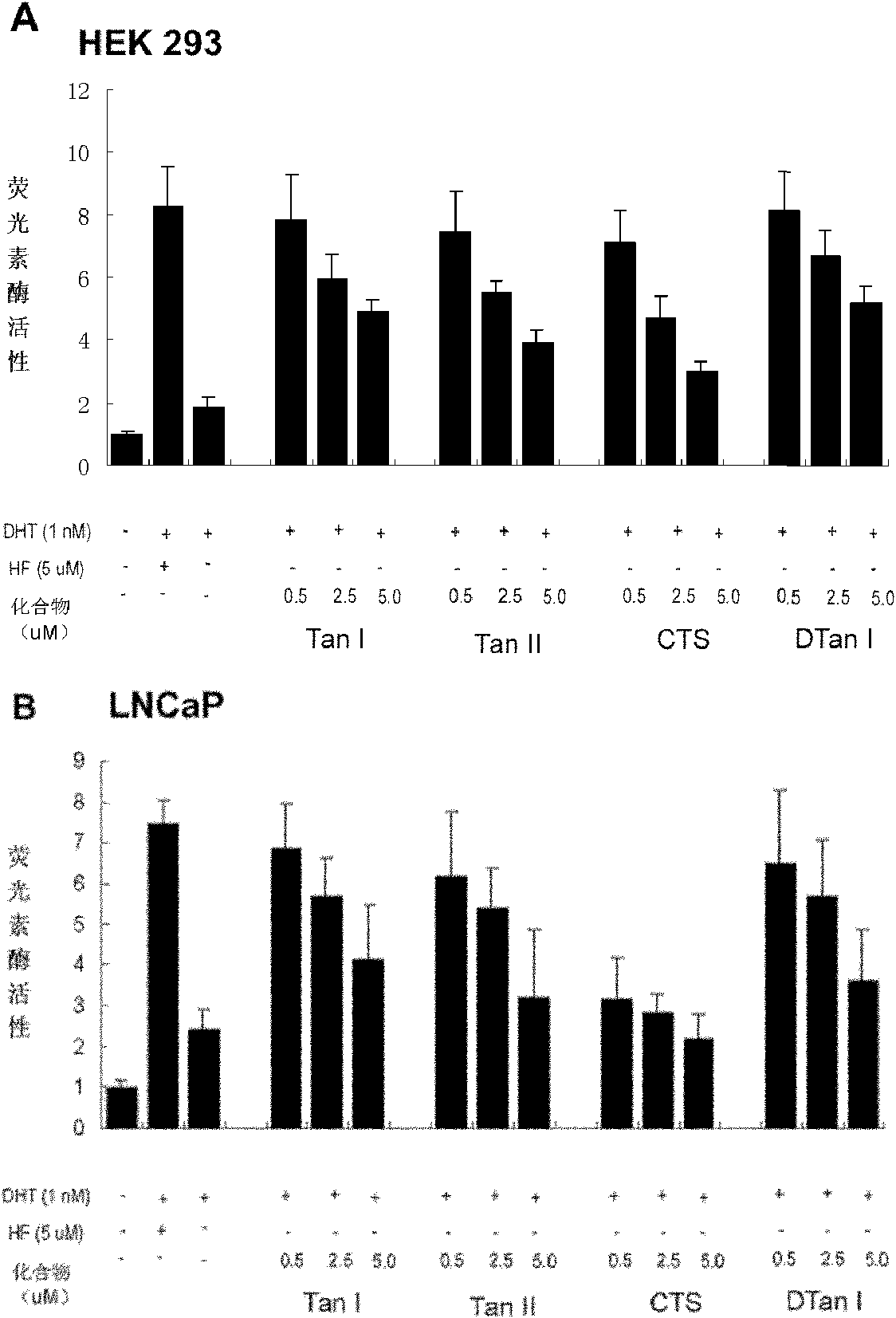
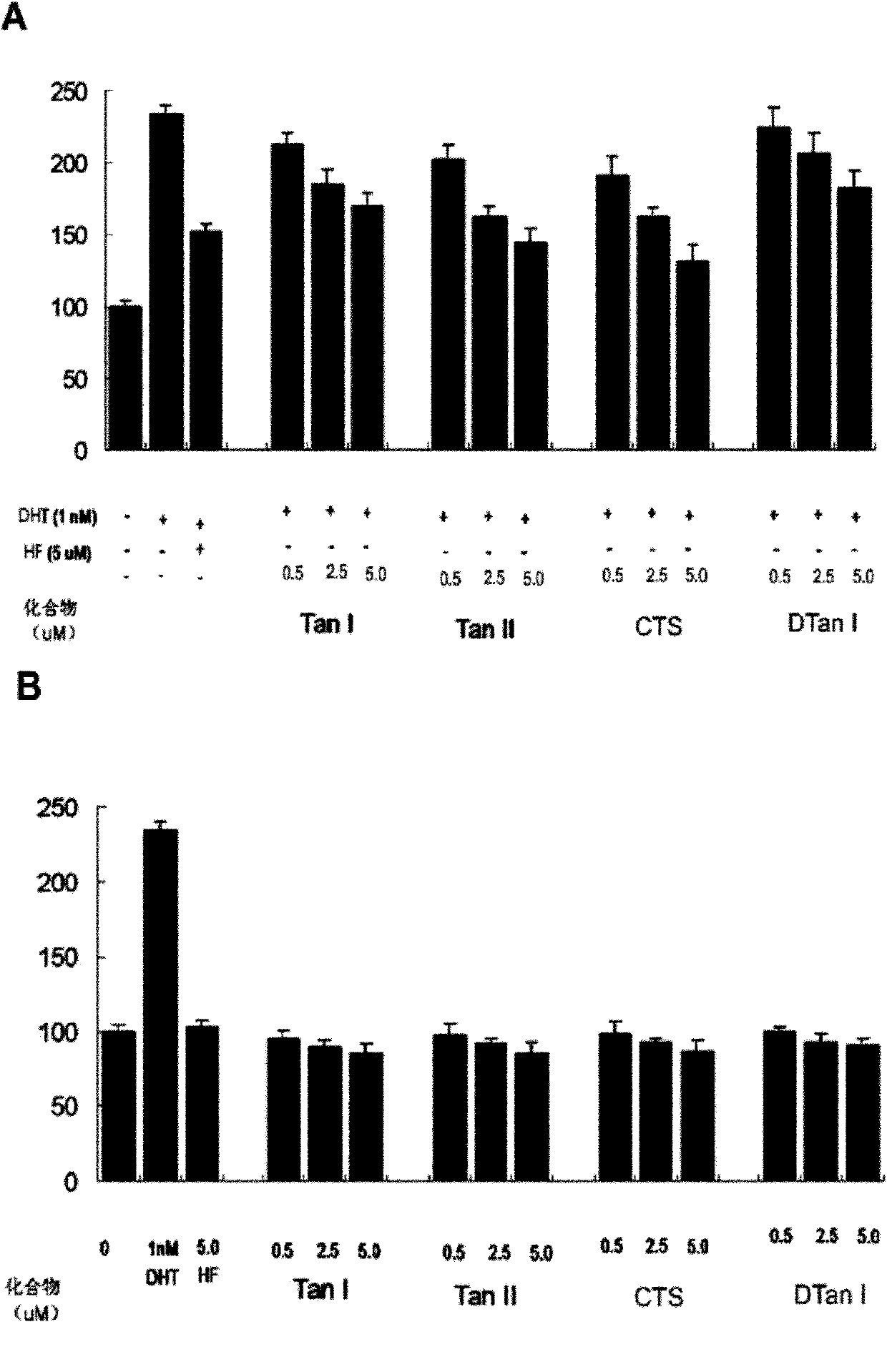
[0028]DHT-regulated transcriptional activity inhibition analysis (AR transactivation assay.) (transfection of wild-type androgen receptor AR expression plasmid)
[0029] Inhibition assays of DHT-regulated transcriptional activity were determined using a Luciferase assay.
[0030] Cell culture and transfection. HEK 293 cells are human embryonic kidney cells, which are relatively easy to transfect. They are a commonly used cell line for expressing foreign genes. Due to the lack of androgen receptor AR, we used wild-type androgen receptor AR expression plasmids, reporter gene plasmids and The mouse mammary tumor virus vector plasmid (MMTV-Luc) was transfected into HEK 293 cells, and Lipofectamin 2000 was used for transfection according to the steps provided by the company (Invitrogen). The experimental process includes the following contents: the 2×10 4 HEK 293 cells were inoculated in 24-well cell culture plates, cultured at 37°C for 24 hours with DMEM medium (100 units / ml pe...
Embodiment 3
[0032] Inhibition analysis of DHT-regulated transcriptional activity II (mutant androgen receptor AR expression plasmid)
[0033] Prostate cancer cell line LNCaP is an androgen-sensitive human prostate cancer cell line. Its androgen receptor AR has been mutated (T877A), and it is expressed as an intrinsic mutant androgen receptor AR. LNCaP cells only need to use mouse mammary tumor virus Vector (MMTV-Luc) and reporter gene plasmid (pRL-TK-luc) were co-transfected to test the activity of tanshinone compounds. The experimental process includes the following: 5×10 4 LNCaP cells were seeded in a 24-well cell culture dish, cultured at 37°C for 24 hours with RPMI-1640 medium (100 units / ml penicillin, 100 mg / L streptomycin, and 10% fetal bovine serum), and DNA lipid Complex MMTV-Luc and pRL-TK-luc were added to the Lipofectamin 2000 reagent for plasmid co-transfection. After 24 hours of transfection, fresh RPMI-1640 10% activated carbon-desorbed serum medium was replaced, and the ce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com