Faropenem sodium freeze-dried powder injection
A technology of faropenem sodium and freeze-dried powder injection, which is applied in the field of pharmaceutical preparations, can solve the problems of unremoved free water, poor solution stability, long production cycle, etc., and achieves a simple and feasible preparation process, high preparation stability, and convenient operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
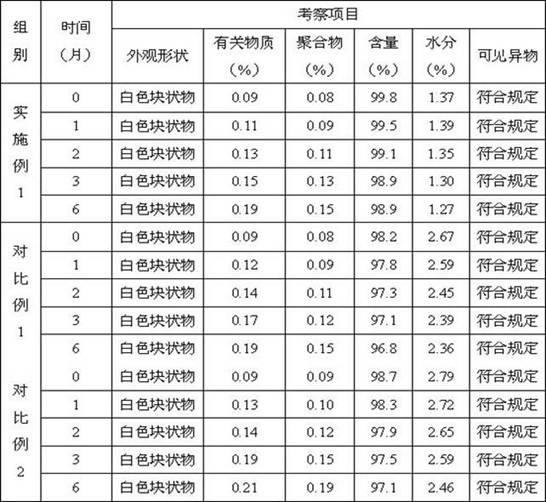
Image
Examples
Embodiment 1
[0021] Faro Peinan sodium 5G
[0022] 100ml of injection water
[0023] The particle size obtained by micro -powder is 5g of sodium powder with a particle size of 75 ~ 100 μm. Add 100ml of injection of water for injection and stir to fully dissolve it. Each 4ML is filled in 10ml Xilin bottle.The frozen dried machine layer layer to -45 ° C. After the filling is over, the Xilin bottle is placed in the frozen dryer and the insulation is 2h.The temperature of the plate layer is heated to -12 ° C, and after 2H, heat the plate to -40 ° C, and continue to keep the insulation for 3h.The plate layer is heated to 20 ° C within 8 ~ 12h; after 8H at 35 ° C, rinse the nitrogen soya, and remove the rolling cover packaging.
Embodiment 2
[0040] [Content measurement] Based on the high -efficiency liquid chromatography method (China Pharmacopoeia 2010 version of the second appendix ⅴ D) determination.
[0041] Chromatographic conditions and system applicability tests are filled with ectopane kel silicone silicone;The ammonium aquatic solution (15: 85: 2) is a flowing phase, and the detection wavelength is 306nm; the number of theoretical tower plates is calculated according to the Fa Luo Peinan Peak not less than 2000.
[0042] The measurement method takes the appropriate amount of this product (about 200mg of Faro Peinan), put it in a 100ml measuring bottle, and dissolve and dilute it to the scale, shake it, filter, and the precision amount is 5ml of 5ml to place 50ml of bottle, add the flow to the flow, add flowingDilute to the scale, shake well as the test product solution, and the precision amount is 20 μL into the liquid chromatography to record the chromatography.In addition, the appropriate amount of sodium c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com