Novel method for preparing aromatic amine through halogenated aromatic hydrocarbon
A technology for halogenated aromatic hydrocarbons and aromatic amines, applied in the field of preparing aromatic amines, can solve the problems of difficult catalyst separation and difficult purification of products, and achieve the effects of wide application range, convenient use and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
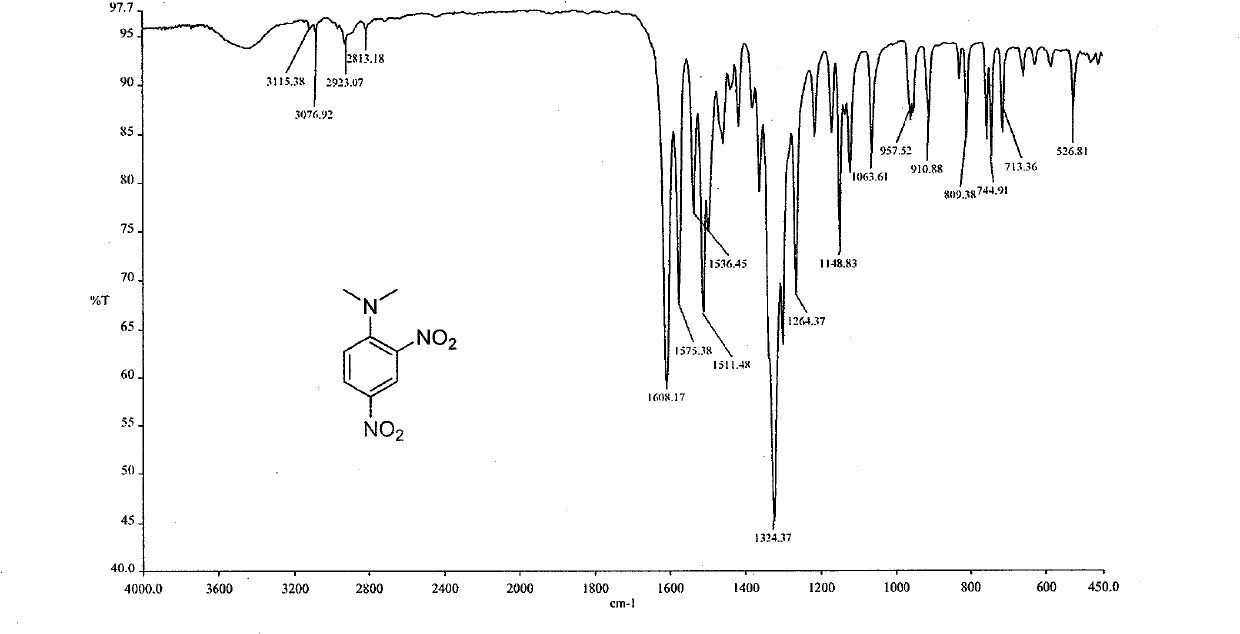
[0052] Add 20ml of dried dimethyl sulfoxide (DMSO) in a 50ml single-mouth bottle, add 3mmol2,4-dinitrochlorobenzene under stirring, then add 8mmol potassium carbonate, 2mmol dimethylamine zinc chloride complex ( [Me 2 NH 2 ] 2 ZnCl 4 ), the mixed solution was stirred and reacted at 60° C. for 10 min. After the reaction was finished, it was cooled, and the reaction solution was dripped dropwise into 80 ml of 2% aqueous sodium hydroxide solution, extracted 3 times with 20 ml of chloroform, washed once with 10 ml of water, dried, and spun The crude product was evaporated, and recrystallized from a mixed solvent of chloroform-n-hexane to obtain N,N-dimethyl-2,4-dinitroaniline (I), with a yield of 96%, Mp 86-88°C.
[0053] The reaction formula of 2,4-dinitrochlorobenzene and dimethylamine zinc chloride complex is:
[0054]
[0055] The spectral data of product (I) is: IR (KBr) v: 3110,1608,1575,1536,1511,1324cm -1 .
Embodiment 2
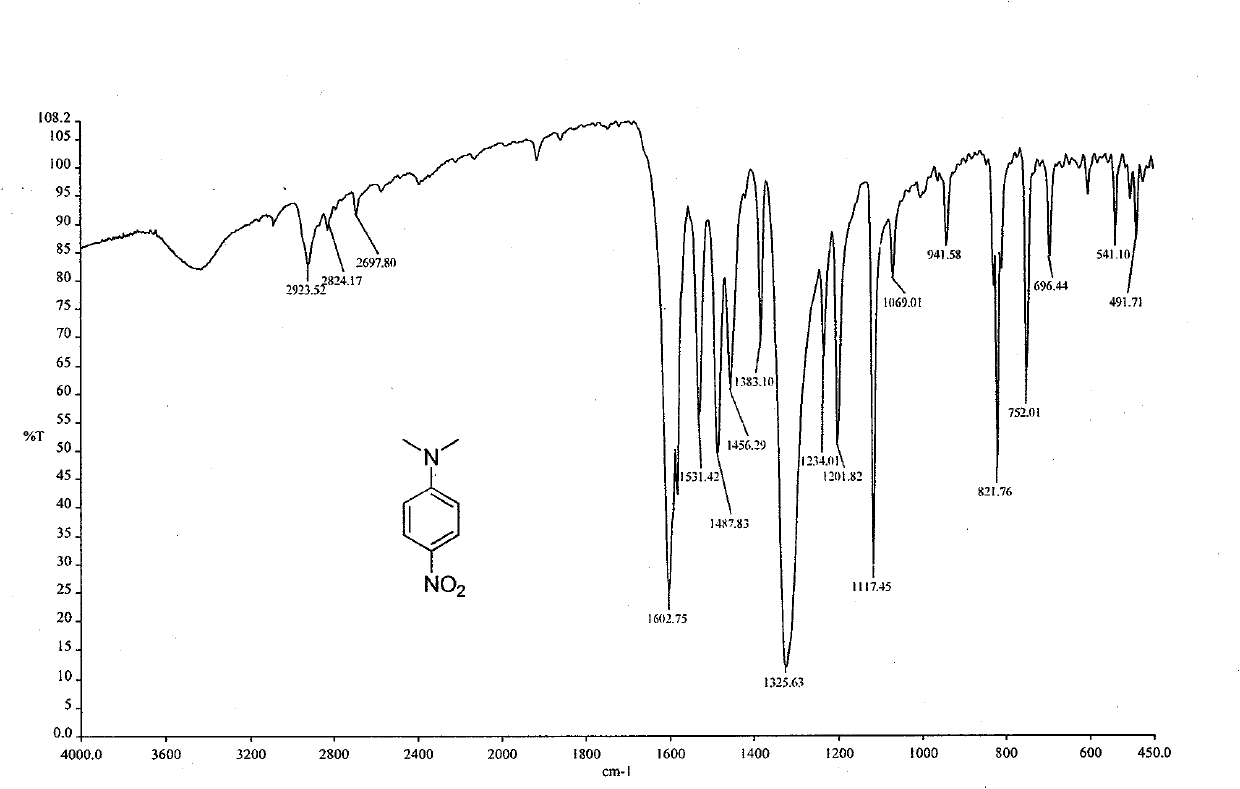
[0057] Replace 2,4-dinitrochlorobenzene with p-nitrochlorobenzene, increase the reaction temperature to 120°C, and react for 5 hours. Others are the same as in Example 1 to obtain the target compound N,N-dimethyl-4-nitroaniline (II), yield 86%, Mp 163-166°C. The reaction formula of p-nitrochlorobenzene and dimethylamine zinc chloride complex is:
[0058]
[0059] The spectral data of the product (II) are: IR(KBr) v: 3110, 1603, 1531, 1488, 1324, 1117, 822, 752.
Embodiment 3
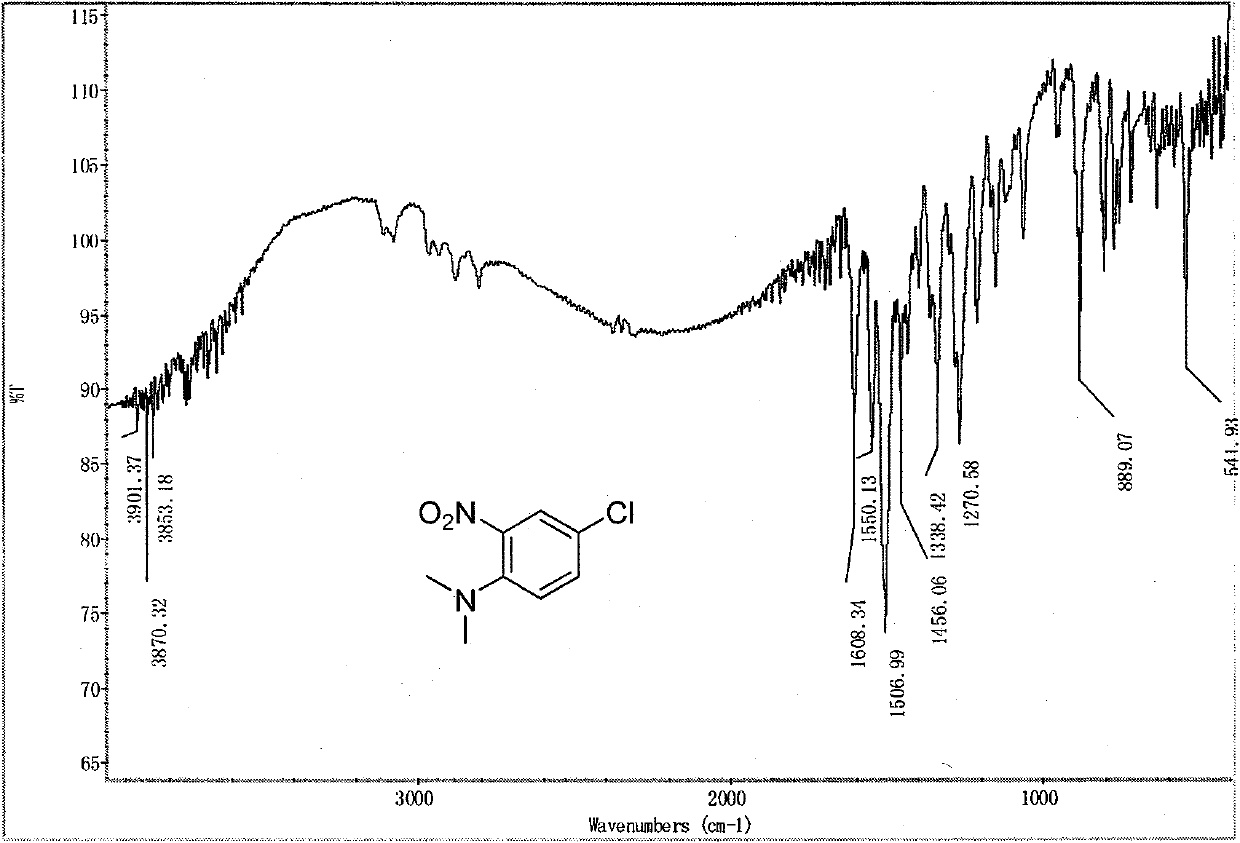
[0061] Replace 2,4-dinitrochlorobenzene with 2,5-dichloronitrobenzene, raise the reaction temperature to 120°C, react for 1h, and the others are the same as in Example 1 to obtain the target compound 4-chloro-N,N-dichlorobenzene Methyl-2-nitroaniline (III), yield 85%, Mp 55-56°C. The reaction formula of 2,5-dichloronitrobenzene and dimethylamine zinc chloride complex is:
[0062]
[0063] The spectral data of product (III) is: IR (KBr) v: 1608, 1550, 1507, 1456, 1338, 1270, 889cm -1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com