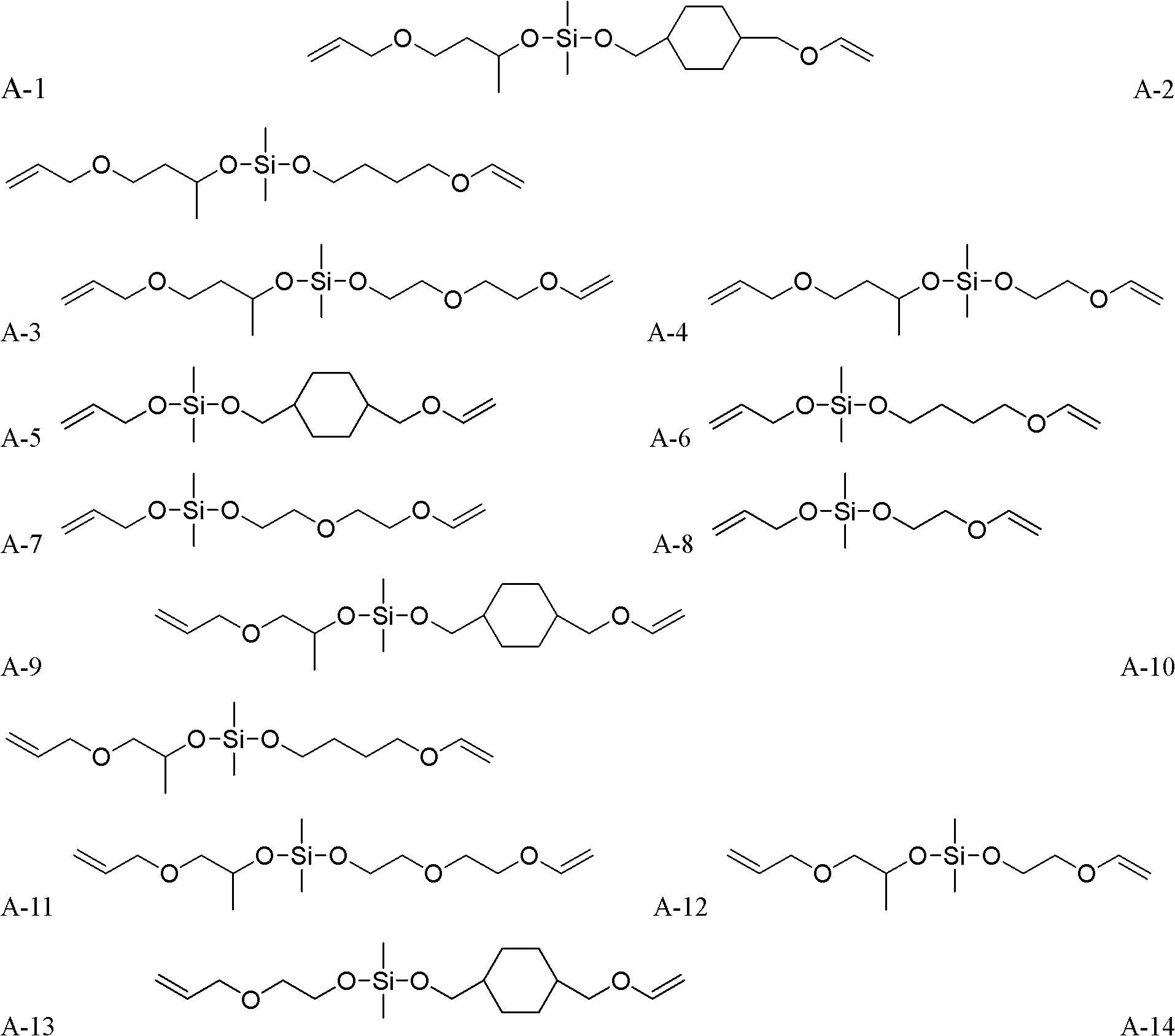Method for synthesizing silicon-containing polymerizing monomer terminated by vinyl ether and allyl ether
The technology of hydroxyalkyl vinyl ether and vinyl ether alkoxy is applied in the field of synthesis of silicon-containing polymerized monomers, and can solve the problems of serious oxygen inhibition of radical photopolymerization, poor surface curing, and difficult performance adjustment, etc. Achieve the effect of improving dry etching resistance, good curing rate, convenient storage and transportation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
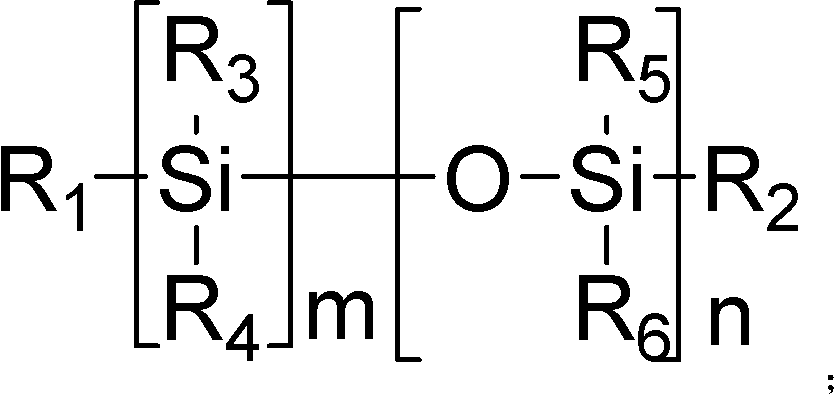
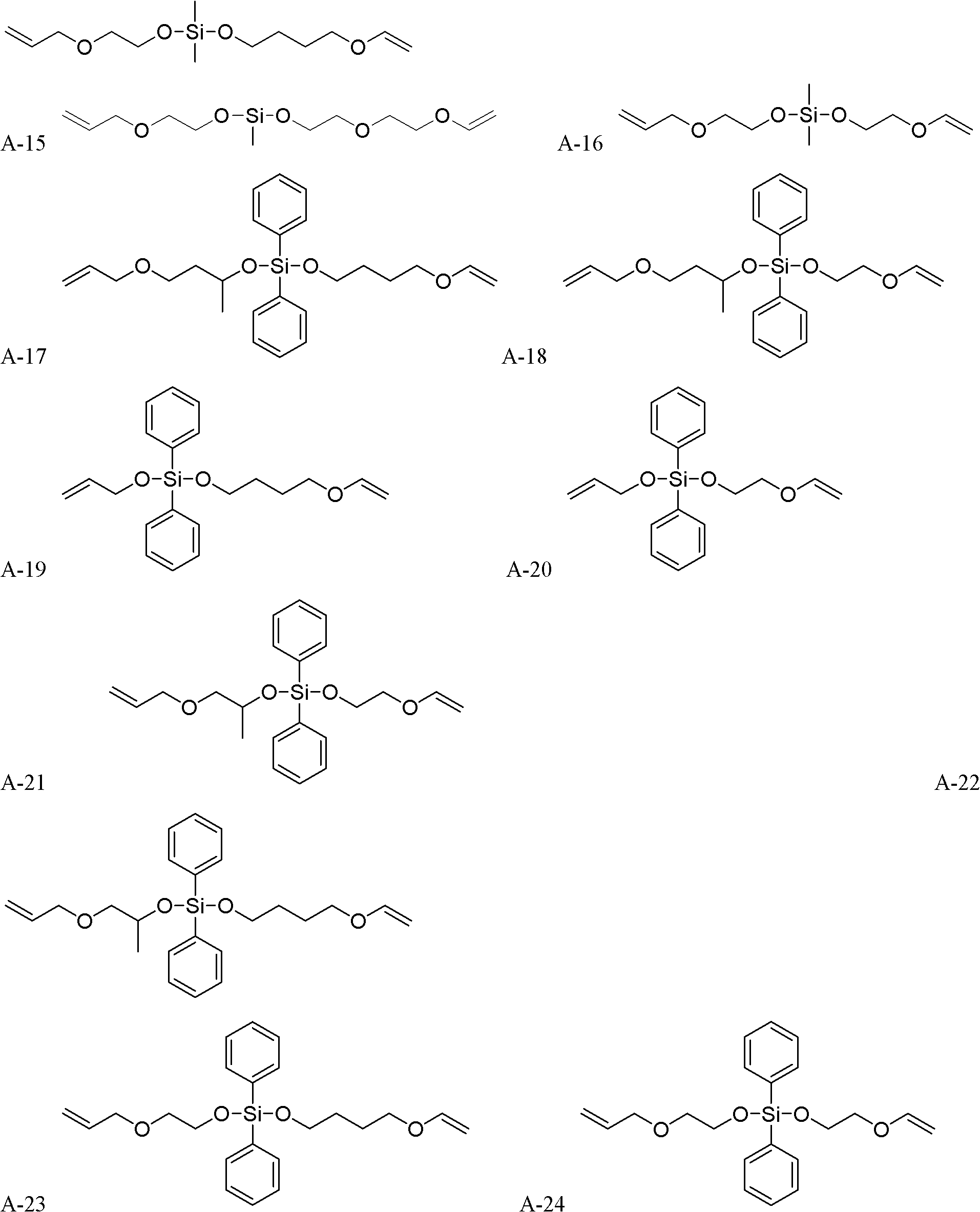
[0049] monomer That is, the synthesis of 1-allyloxy-1-vinyloxybutoxydimethylsilane:
[0050] Add 17.8202g (0.176mol) of triethylamine and 120mL of tetrahydrofuran into a 500mL four-necked flask, pass N 2 Stir in an ice-water bath to cool it down; dissolve 9.2935g (0.08mol) of 4-hydroxybutyl vinyl ether and 3.6645g (0.132mol) of allyl alcohol in 100mL of tetrahydrofuran; dissolve 10.3222g (0.08mol) of dimethyl di Chlorosilane was quickly added to the four-necked bottle, and the two alcohols were added dropwise at a rate of about 4 drops / s, and the drop was completed in about 20 minutes; after the drop, it became white and turbid, removed the ice-water bath, and stirred at room temperature for 1.5 hours; the reaction was completed After suction filtration, the white solid was filtered off, the filtrate was a colorless transparent liquid, and THF was removed by rotary evaporation; extracted with 80mL of n-hexane, filtered by suction, the filtrate was a colorless transparent liq...
Embodiment 2
[0052] monomer That is, the synthesis of 1-allyl-1-[2-(2-vinyloxy)ethoxyethoxy]dimethylsilane:
[0053] Diethylene glycol monovinyl ether was used to replace 4-hydroxybutyl vinyl ether in Example 1, and the remaining reagents and amounts were the same as in Example 1.
Embodiment 3
[0055] monomer That is, the synthesis of 1-(2-allyl)ethoxy-1-vinyloxybutoxydimethylsilane:
[0056]Allyl hydroxy vinyl ether is used to replace the allyl alcohol in Example 1, and the remaining reagents and consumption are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com