Method for producing hydrogen by reforming methanol steam
A technology for reforming hydrogen and methanol, applied in chemical instruments and methods, hydrogen, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problem of low hydrogen production activity, easy crystal growth, and unspecified composition of the system and preparation methods to achieve low CO selectivity, improved catalyst stability, and long-life operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
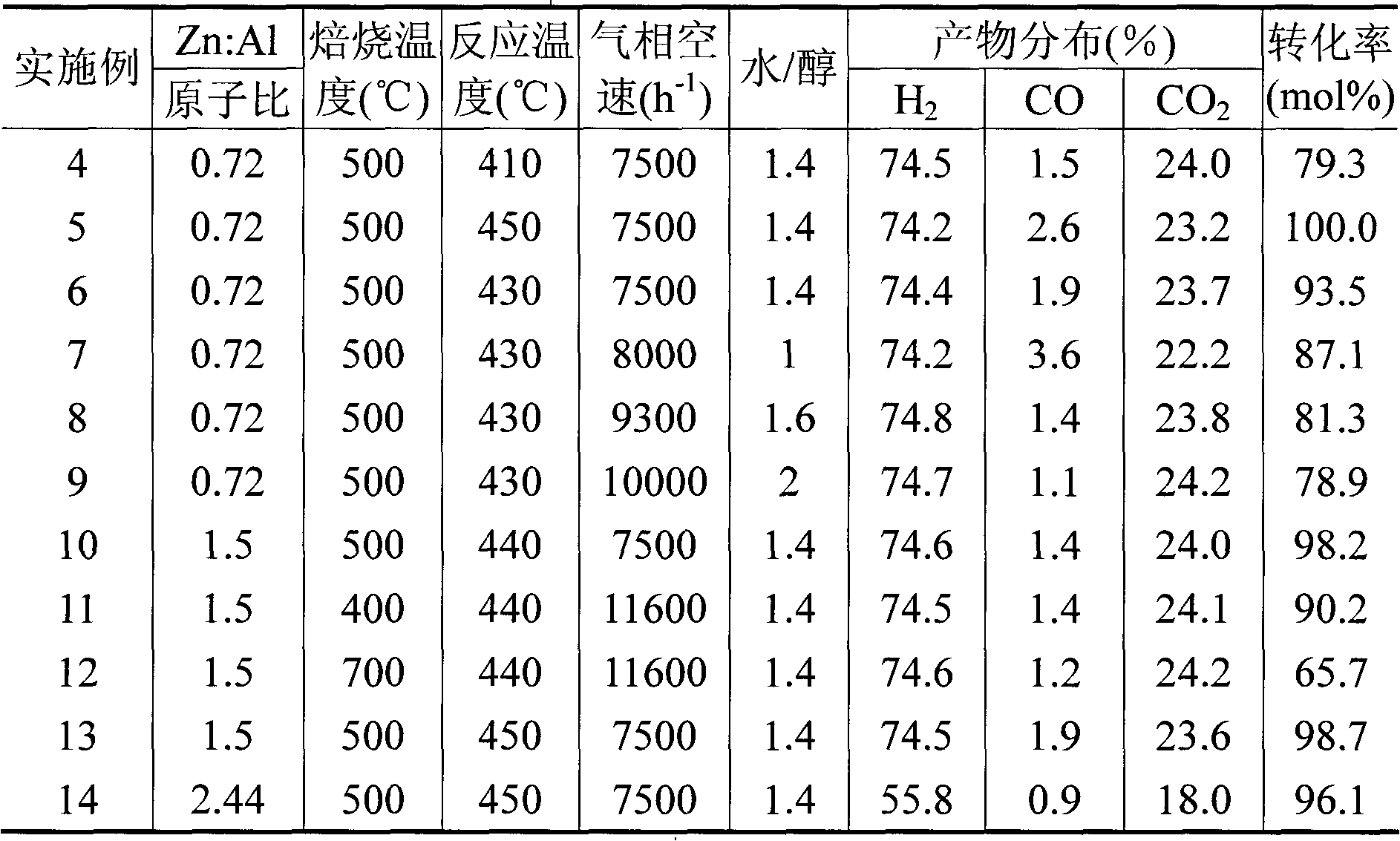
[0020] Catalyst preparation (co-precipitation + impregnation): according to zinc-aluminum spinel (ZnAl 2 o 4 ) stoichiometric ratio of the required nitrate into 1M aqueous solution and 1M ammonia water and flow in the sedimentation tank, fully stirred, titration rate 10ml / min, keep pH=7.5, precipitation is complete, aging, suction filtration, washing, drying Dry, roast at 500°C for 4 hours to make spinel powder with a particle size of 5-15nm, impregnate ZnAl with zinc nitrate aqueous solution 2 o 4 Powder, made of ZnO / ZnAl with a particle size of 10-30nm according to the zinc-aluminum atomic ratio of 0.56:1 2 o 4 catalyst.
[0021] The prepared ZnO / ZnAl 2 o 4 The catalyst powder is pressed into tablets and sieved into 40-60 meshes, put into a fixed-bed microreactor, the reaction temperature is 430°C, and the raw material H 2 O / CH 3OH=1.4 (molar ratio) after mixing, it is first pumped into the vaporization system and then into the reactor, and the gas phase space veloci...
Embodiment 2
[0023] Catalyst preparation (co-precipitation method): Weigh the required amount of nitrate according to the zinc-aluminum atomic ratio of 0.72:1, make 1M aqueous solution and 1M ammonia water and flow in the precipitation tank, stir well, and keep pH = 7; after the precipitation is complete , aged for 1 hour, filtered and washed, dried at 110°C, and calcined at 500°C for 4 hours.
[0024] The prepared ZnO / ZnAl 2 o 4 The catalyst powder is pressed into tablets and sieved into 40-60 meshes, put into a fixed-bed microreactor, the reaction temperature is 440°C, and the raw material H 2 O / CH 3 OH=1.4 (molar ratio) after mixing, it is first pumped into the vaporization system and then into the reactor, and the gas phase space velocity is 7500h -1 , reaction under normal pressure, methanol conversion rate 99.2%, H 2 , CO molar concentrations were 74.4%, 2.15%.
Embodiment 3
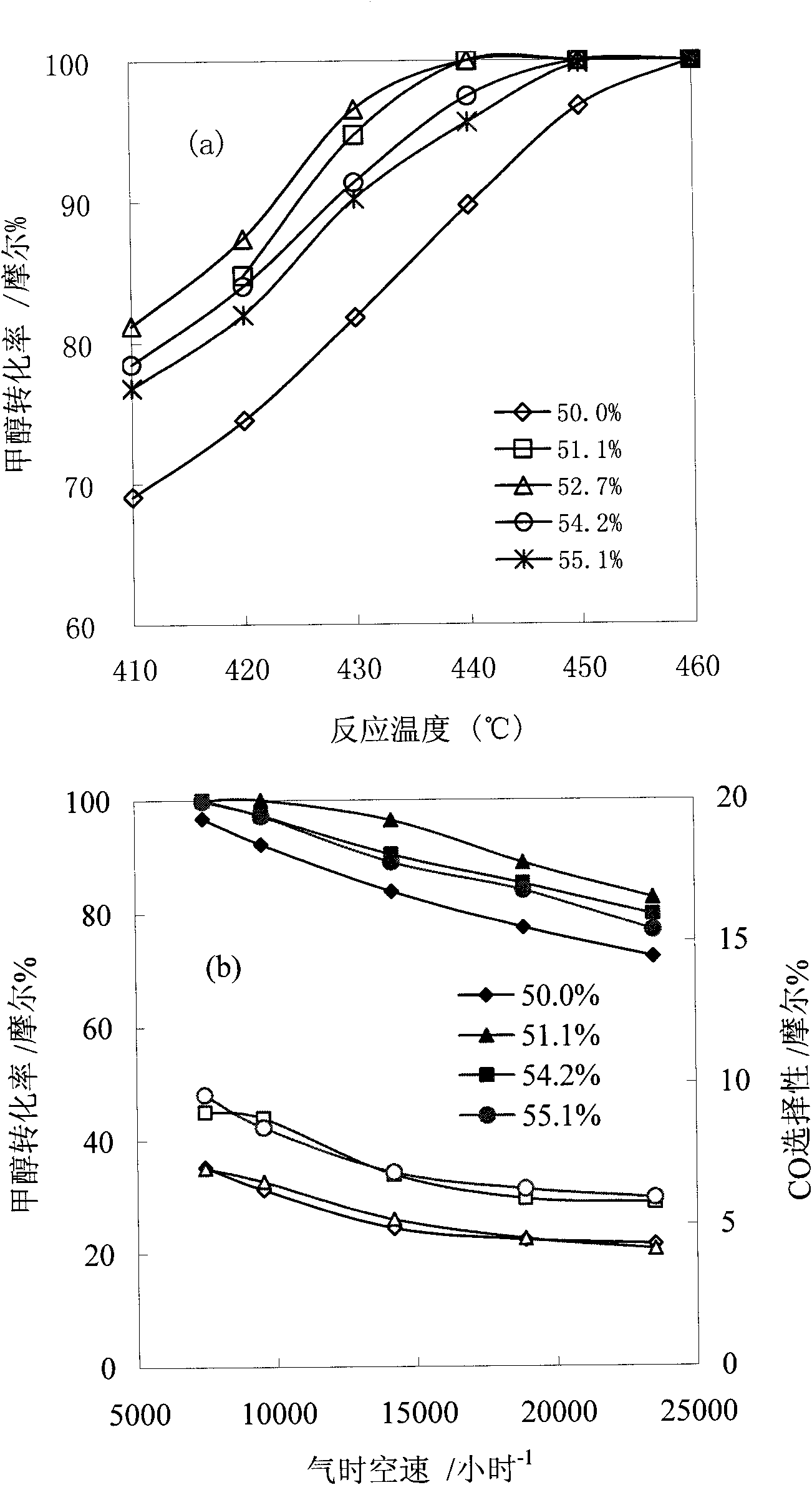
[0026] Operate according to each step of Example 1, change the zinc-aluminum atomic ratio 0.5: 1 (that is, only co-precipitate to generate the zinc aluminate of stoichiometric ratio, without impregnation step), 0.52: 1, 0.59: 1, 0.61: 1, change the reaction Temperature from 410-460°C, fixed reaction space velocity 7400h -1 And the water-alcohol molar ratio is 1.4, the catalytic methanol steam reforming activity results are shown in the appendix figure 1 (a).
[0027] Operate according to each step of Example 1, change the zinc-aluminum atomic ratio 0.5: 1 (that is, only co-precipitate to generate the zinc aluminate of stoichiometric ratio, without impregnation step), 0.52: 1, 0.59: 1, 0.61: 1, change the reaction Empty from 7400-24000h -1 , with a fixed reaction temperature of 450°C and a water-alcohol molar ratio of 1.4, the catalytic methanol steam reforming activity results are shown in the appendix figure 1 (b).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com