Method for preparing lithium iron phosphate in ion liquid
A lithium iron phosphate, ionic liquid technology, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry and other directions, can solve the problems of insufficient electrochemical activity, increased cost input, large particle size of materials, etc., to meet the requirements of reducing equipment, The effect of reducing production cost and high crystallinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
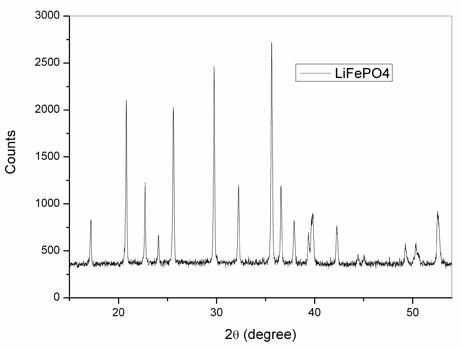
Image
Examples
Embodiment 1
[0026] A method for preparing lithium ferrous phosphate in an ionic liquid uses the ionic liquid as a reaction medium and completes it through a soft chemical synthesis method.
[0027] The ionic liquid is composed of choline chloride and urea.
[0028] The steps of the soft chemical synthesis method include:
[0029] 1) Add lithium nitrate, ferrous sulfate and triethyl phosphate into the ionic liquid, stir well to mix the raw materials evenly, exhaust the air in the reaction tank with nitrogen, and react at a constant temperature of 140°C for 96 hours;
[0030] 3) The reaction product is washed with water several times and then dried to obtain nanoscale lithium iron phosphate that can be applied to lithium ion batteries.
[0031] The addition amount of described lithium nitrate, ferrous sulfate and triethyl phosphate is 1mmol.
[0032] The ionic liquid is uniformly mixed with choline chloride and urea with a molar ratio of 1:5, and dried at 50° C. to obtain the ionic liquid...
Embodiment 2
[0036] A method for preparing lithium ferrous phosphate in an ionic liquid uses the ionic liquid as a reaction medium and completes it through a soft chemical synthesis method.
[0037] The ionic liquid is composed of choline chloride and urea.
[0038] The steps of the soft chemical synthesis method include:
[0039] 1) Add lithium nitrate, ferrous sulfate and triethyl phosphate into the ionic liquid, stir well to mix the raw materials evenly, exhaust the air in the reaction tank with nitrogen, and react at a constant temperature of 250°C for 12 hours;
[0040] 3) The reaction product is washed with water several times and then dried to obtain nanoscale lithium iron phosphate that can be applied to lithium ion batteries.
[0041] The addition amount of described lithium nitrate, ferrous sulfate and triethyl phosphate is 10 mmol.
[0042] The ionic liquid is uniformly mixed with choline chloride and urea with a molar ratio of 5:1, and dried at 200° C. to obtain the ionic liq...
Embodiment 3
[0046] A method for preparing lithium ferrous phosphate in an ionic liquid uses the ionic liquid as a reaction medium and completes it through a soft chemical synthesis method.
[0047] The ionic liquid is composed of choline chloride and urea.
[0048] The steps of the soft chemical synthesis method include:
[0049] 1) Add lithium nitrate, ferrous sulfate and triethyl phosphate into the ionic liquid, stir well to mix the raw materials evenly, exhaust the air in the reaction tank with nitrogen, and react at a constant temperature of 220 °C for 48 h;
[0050] 3) The reaction product is washed with water several times and then dried to obtain nanoscale lithium iron phosphate that can be applied to lithium ion batteries.
[0051] The addition amount of described lithium nitrate, ferrous sulfate and triethyl phosphate is 5mmol.
[0052] The ionic liquid is uniformly mixed with choline chloride and urea in a molar ratio of 3:1, and dried at 150° C. to obtain the ionic liquid use...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com