Method for preparing anhydrous magnesium chloride for electrolyzing magnesium
A technology of anhydrous magnesium chloride and electrolytic magnesium, which is applied in the direction of magnesium chloride and magnesium halide, can solve the problems of industrialization difficulties and low ammonium utilization rate, and achieve the effects of cost reduction, low cost, and simple preparation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
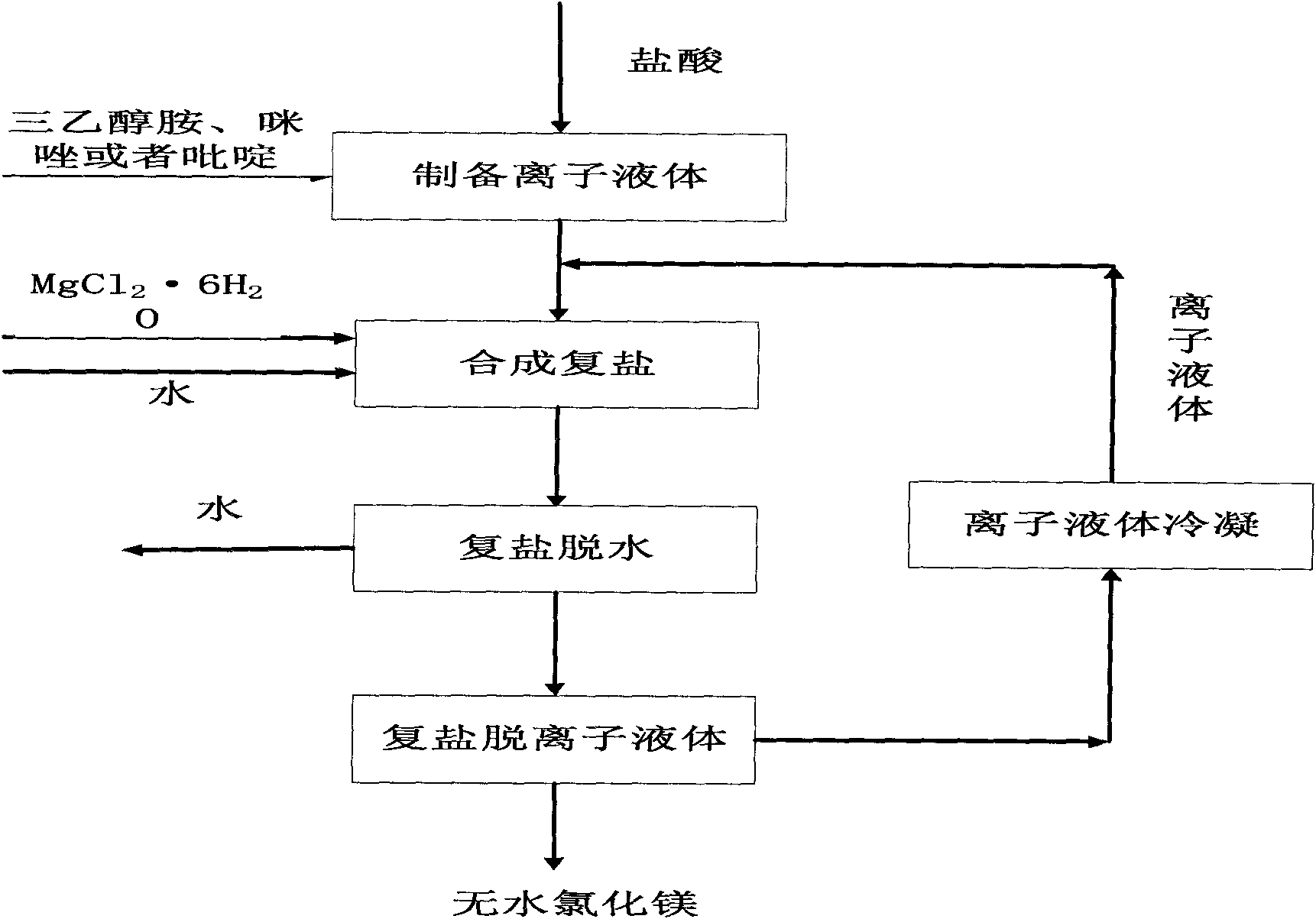
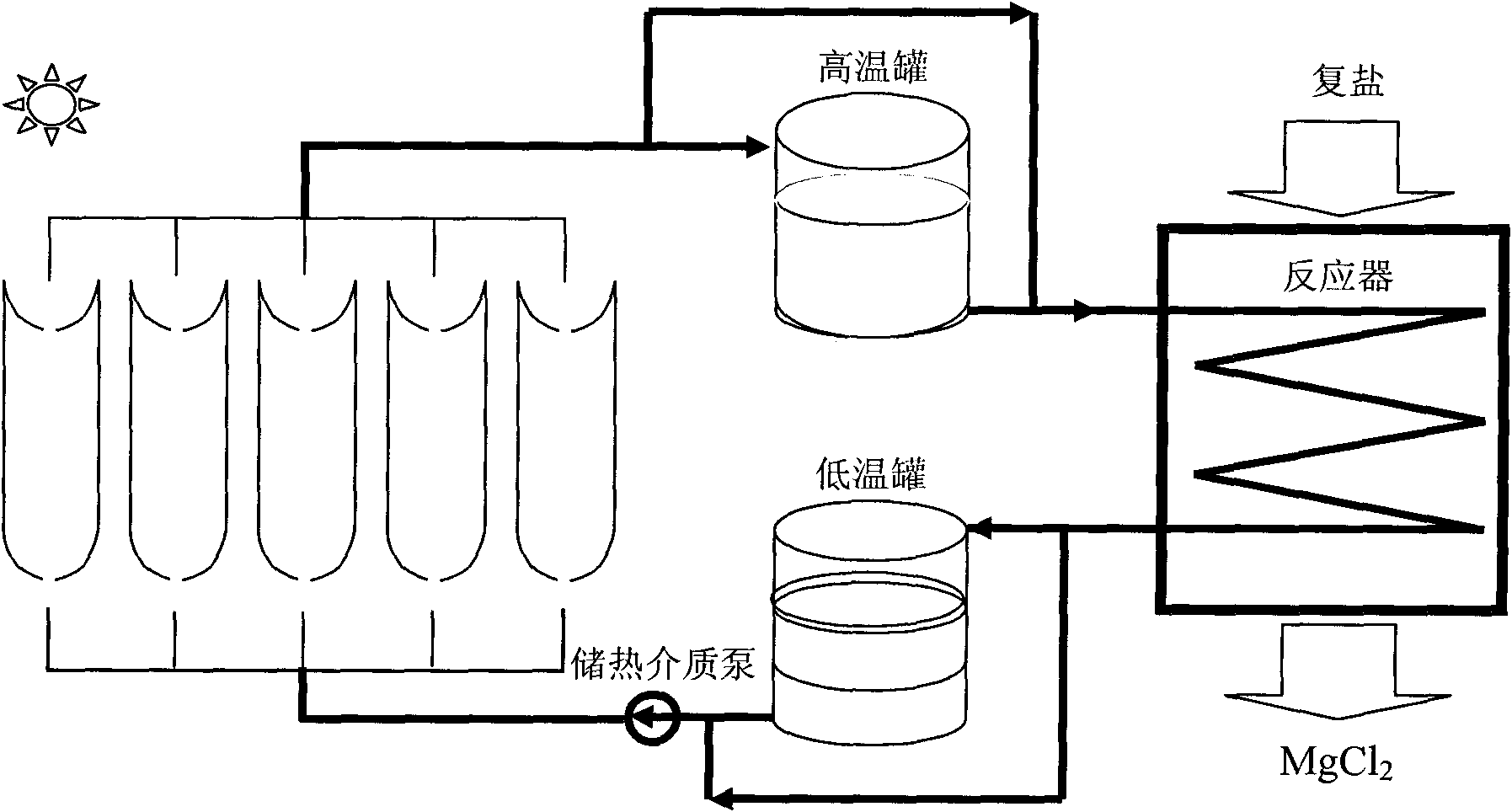
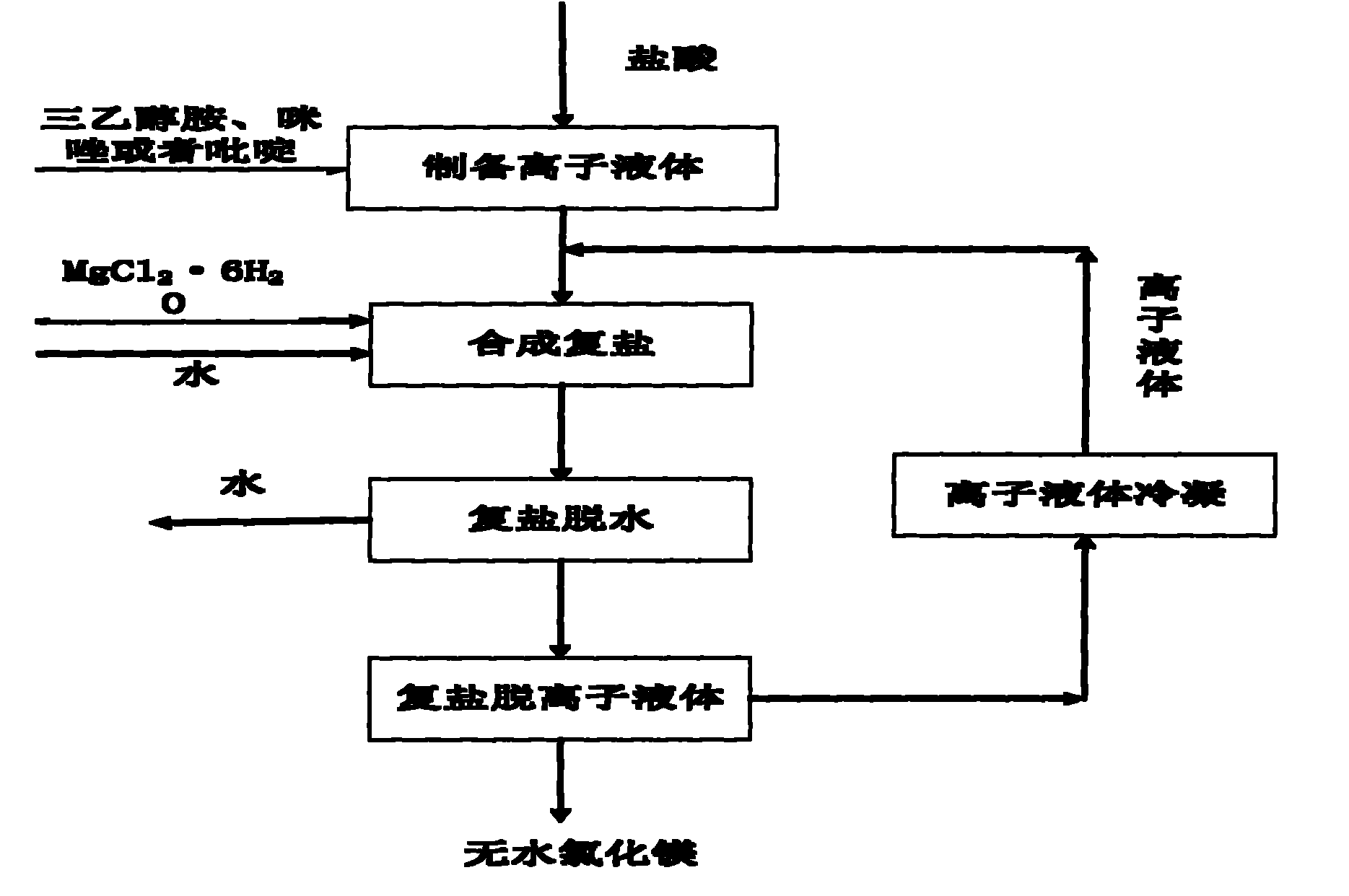
[0039] See figure 1 and figure 2 .
[0040] 1) Take 560 grams of triethanolamine and add it to the reaction crystallization kettle, control the temperature of the reaction kettle to 30°C, and slowly add 380.54 grams of industrial hydrochloric acid dropwise to the reaction crystallization kettle while stirring; the reaction time is 2 hours later , the crystal slurry is filtered, and the obtained solid is [HTEA]Cl ionic liquid crude product;
[0041] 2) Drying the crude [HTEA]Cl ionic liquid obtained in step 1) in a drying oven at 50°C for 8 hours to obtain the [HTEA]Cl ionic liquid;
[0042] 3) Add 203.3 grams of MgCl to the reaction crystallization kettle 2 ·6H 2 O, slowly heat up to 100°C, add 3.6 grams of water, stir to make it fully dissolved, then take 185.69 grams of [HTEA]Cl ionic liquid obtained in step 2) and add it to the reaction crystallization kettle, stir and dissolve to form a homogeneous phase, and then naturally cool to room temperature , the obtained cry...
Embodiment 2
[0046] See figure 1 and figure 2 .
[0047] 1) Take 560 grams of triethanolamine and add it to the reaction crystallization kettle, control the temperature of the reaction kettle to be 10°C, and slowly add 360.54 grams of hydrochloric acid dropwise to the reaction crystallization kettle while stirring; after the reaction time is 2 hours, The crystal slurry is filtered, and the obtained solid is [HTEA]Cl ionic liquid crude product;
[0048] 2) The crude [HTEA]Cl ionic liquid obtained in step 1) was washed twice with absolute ethanol, and then dried in a drying oven at 60°C for 5 hours to obtain [HTEA]Cl ionic liquid with a purity of 98.5%;
[0049] 3) Add 203.3 grams of MgCl to the reaction crystallization kettle 2 ·6H 2 O, slowly heat up to 100°C, add 3.6 grams of water, stir to make it fully dissolved, then take 185.69 grams of the [HTEA]Cl ionic liquid obtained in step 2) and add it to the reaction crystallization kettle, stir and dissolve to form a homogeneous phase, t...
Embodiment 3
[0053] See figure 1 and figure 2 .
[0054] 1) Add 82 grams of imidazole into the reaction crystallization kettle, control the temperature of the reaction kettle to 40°C, and slowly add 105.66 grams of hydrochloric acid dropwise into the reaction crystallization kettle while stirring; after the reaction time is 1 hour, the crystal slurry Filtration, the obtained solid is [HIM]Cl ionic liquid crude product;
[0055] 2) The crude [HIM]Cl ionic liquid obtained in step 1) was washed twice with absolute ethanol, and dried in a drying oven at 40°C for 10 hours to obtain [HIM]Cl ionic liquid with a purity of 99.0%;
[0056] 3) Add 203.3 grams of MgCl to the reaction crystallization kettle 2 ·6H 2 O, slowly warming up to 90°C, then adding 118.5 grams of [HIM]Cl ionic liquid obtained in step 2) into the reaction crystallization kettle, adding 1.8 grams of deionized water, stirring and dissolving into a homogeneous phase, and then naturally cooling to room temperature, the obtained...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com