Catalytic polymerization method for lactide
A technology of catalytic polymerization and lactide, which is applied in the field of catalytic polymerization of lactide, can solve the problems of complex structure of rare earth alkoxy complexes, unfavorable catalyst catalytic performance, structure-activity relationship, etc. The effect of clear center and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
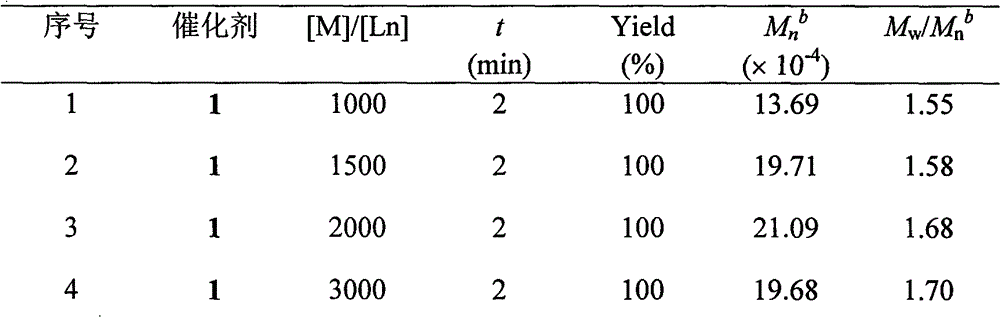
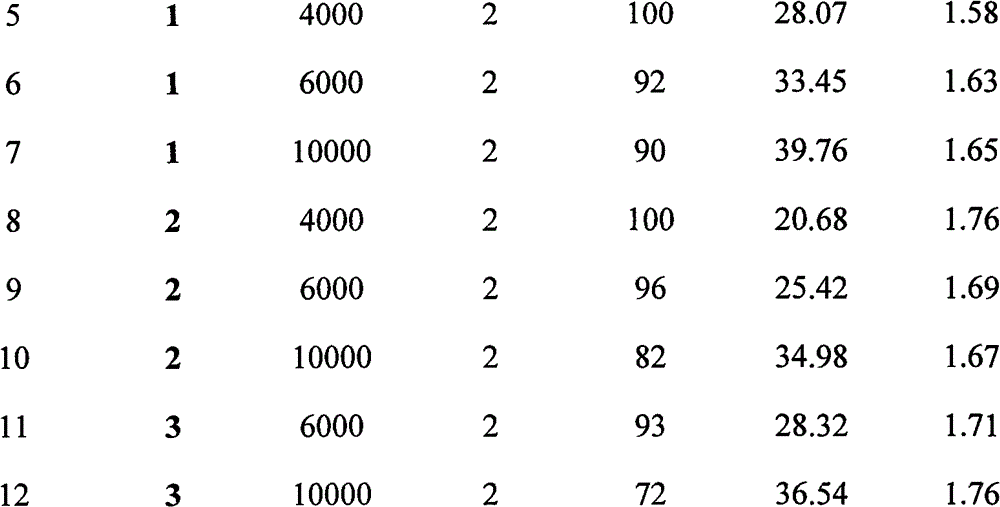
[0025] The rare earth amine complex in this embodiment is a guanidine rare earth diamine complex [(Me 3 Si) 2 NC(NCy) 2 ]Y[N(SiHMe 2 ) 2 ] 2 (THF) (catalyst 1 in the table below), the synthesis process is as follows: adopt Schlenk technique or in a glove box, take 1 mmol of HN(SiMe 3 ) 2 , dissolved in n-hexane, slowly added dropwise to 1mmol n-butyllithium n-hexane solution (concentration: 1.0M) with a volume of 1mL; after stirring for one hour, slowly added dropwise to 1mmol mass of 0.206g N,N'-di In the n-hexane solution of cyclohexylcarbodiimide, react at normal temperature for 0.5 hour; Slowly add dropwise the above-mentioned turbid solution to 1mmol of 0.195g yttrium chloride (YCl 3 ) in THF turbid solution, reacted for 15 minutes; 2mmol of LiN(SiHMe 2 ) 2 Dubbed tetrahydrofuran solution. After reacting at room temperature for 4 hours, the solvent was dried under vacuum, and the residual powder was extracted with n-hexane; the extract was filtered, the supernata...
Embodiment 2
[0028] The rare earth amine complex in this embodiment is a guanidine rare earth diamine complex [(Me 3 Si) 2 NC(NCy) 2 ]Y[N(SiMe 3 ) 2 ] 2 (Catalyst 2 in the table below), its synthesis process is as follows: adopt Schlenk technology or in glove box, take 1mmol quality and be 0.161g HN(SiMe 3 ) 2 , dissolved in n-hexane, slowly added dropwise to 1mmol n-butyllithium n-hexane solution (concentration: 1.0M) with a volume of 1mL; after stirring for one hour, slowly added dropwise to 1mmol mass of 0.206g N,N'-di In the n-hexane solution of cyclohexylcarbodiimide, react at normal temperature for 0.5 hour; Slowly add dropwise the above-mentioned turbid solution to 1mmol of 0.195g yttrium chloride (YCl 3 ) in tetrahydrofuran turbid solution, and reacted for 15 minutes; 2mmol volume of 1.2mL NaN(SiMe 3 ) 2 solution (concentration of 1.67M tetrahydrofuran solution). After reacting at room temperature for 4 hours, the solvent was drained under vacuum, and the residual powder w...
Embodiment 3
[0031] The rare earth amine complex in this embodiment is a guanidine rare earth diamine complex [(Me 3 Si) 2 NC(NCy) 2 ]Lu[N(SiMe 3 ) 2 ] 2 (Catalyst 3 in the table below), its synthesis process is as follows: adopt Schlenk technique or in glove box, take 1mmol quality and be 0.161g HN(SiMe 3 ) 2 , dissolved in n-hexane, slowly added dropwise to 1mmol n-butyllithium n-hexane solution (concentration: 1.0M) with a volume of 1mL; after stirring for one hour, slowly added dropwise to 1mmol mass of 0.206g N,N'-di In the n-hexane solution of cyclohexylcarbodiimide, react at room temperature for 0.5 hours. The above cloudy solution was slowly added dropwise to 1 mmol of 0.281 g of lutetium chloride (LuCl 3 ) in tetrahydrofuran turbid solution, and reacted for 15 minutes; 2mmol volume of 1.2mL NaN(SiMe 3 ) 2solution (concentration of 1.67M tetrahydrofuran solution). After reacting at room temperature for 4 hours, the solvent was drained under vacuum, and the residual powder...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com