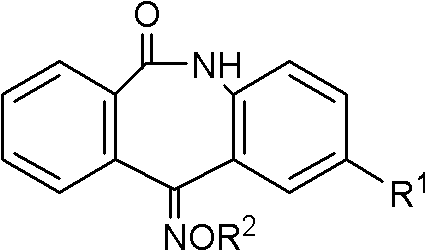
Hydrocarbon-oxygen imino group dibenzo caprolactam derivative, as well as preparation method of the derivative and application of the derivative in serving as bactericide
A fungicide, benzyl technology, applied in the field of alkoxyiminodibenzocaprolactam derivatives and its preparation and application as a fungicide, can solve the problems of long synthetic route, not wide activity spectrum, high cost, etc., and achieve raw material Inexpensive, simple reaction route, good growth inhibition effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1, the synthesis of alkoxyiminodibenzocaprolactam derivatives (CAUWL-2010)
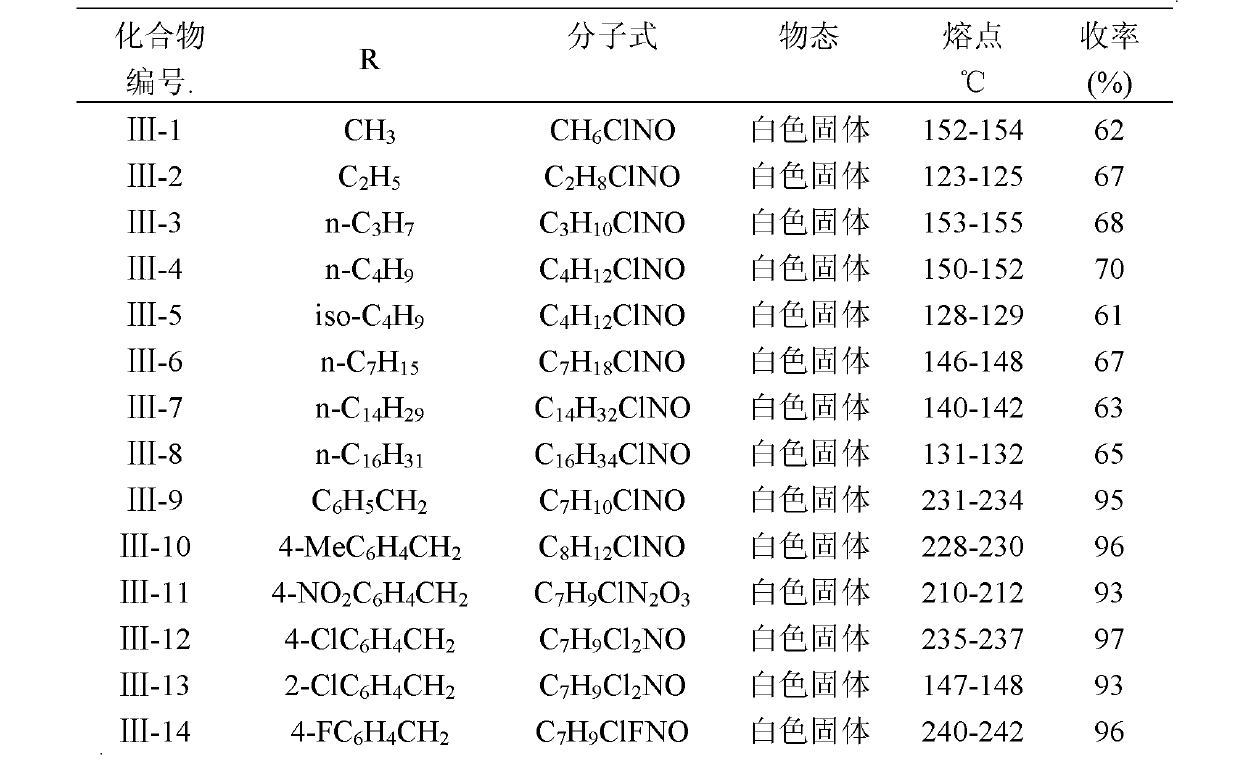
[0059] Add 3mmol of intermediate 1 (R1=H) or intermediate 2 (R1=Cl, Br, I) and 3.6mmol of alkoxyamine hydrochloride into 10mL of pyridine, stir at 90°C for 16 hours, and concentrate the reaction solution to After 2 mL, it was poured into water to produce a white solid, which was washed successively with appropriate amount of dilute hydrochloric acid and water, dried and recrystallized with ethyl acetate and petroleum ether (v / v=1:10) to obtain two isomers (Z and E) mixtures. The existence of cis-trans isomers was confirmed by HPLC-MS, and the ratio was basically 1:1. The physicochemical and mass spectrometry data of the compound CAUWL-2010 are shown in Table 2, and the proton nuclear magnetic resonance spectrum data of the compound CAUWL-2010 are shown in Table 3.
[0060] references:
[0061] Wild, Hanno; Roeben, Wolfgang; Aichinger, Gerd, et al.Preparation of 5,6-dihydro-dibenz[...
Embodiment 2
[0062] Embodiment 2, the preparation of compound CAUWL-2010 emulsifiable concentrate
[0063] Add 1-10g of compound CAUWL-2010, 5-15g of emulsifier, and 0.1-1g of penetrant into a 100ml volumetric flask, and then use solvents such as toluene, xylene, etc. to dilute to obtain an emulsifiable concentrate with a content of 1-10%.
Embodiment 3
[0064] Embodiment 3, the preparation of compound CAUWL-2010 wettable powder
[0065] Take 15-50 g of compound CAUWL-2010, 10-20 g of surfactant, and 30-75 g of white carbon black, mix and pulverize to obtain a wettable powder with a content of 15-50%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com