EV71 virus-like particles and hand-foot-and-mouth disease vaccine prepared from EV71 virus-like particles
A virus-like and particle technology, applied in the field of biomedicine, can solve the problems of restricting large-scale production requirements, complex VLP purification process, and high requirements for culture conditions, achieving reasonable spatial folding, facilitating industrial production, and simplifying the screening process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
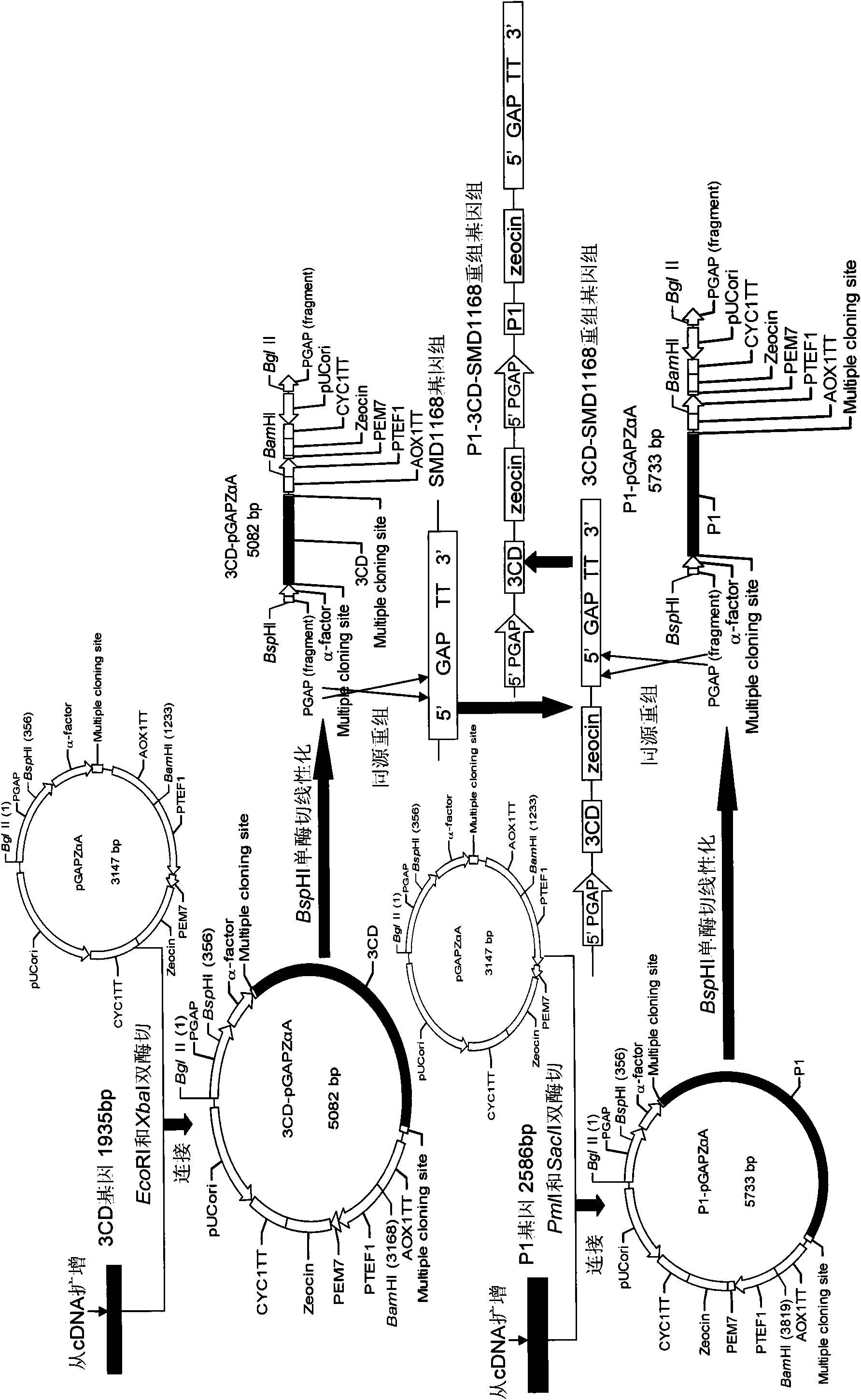
[0034] Embodiment Preparation of EV71 virus-like particles of the present invention
[0035] For the molecular biology experimental methods such as RT-PCR, restriction enzyme digestion and ligation used in the present invention, please refer to the second edition of "Molecular Cloning".
[0036] The basic materials for preparing EV71 virus-like particles of the present invention include: EV71 virus P1 gene, 3CD gene, pGAPZαA plasmid (product of Invitrogen, USA), and yeast strain SMD1168 (product of Invitrogen, USA).
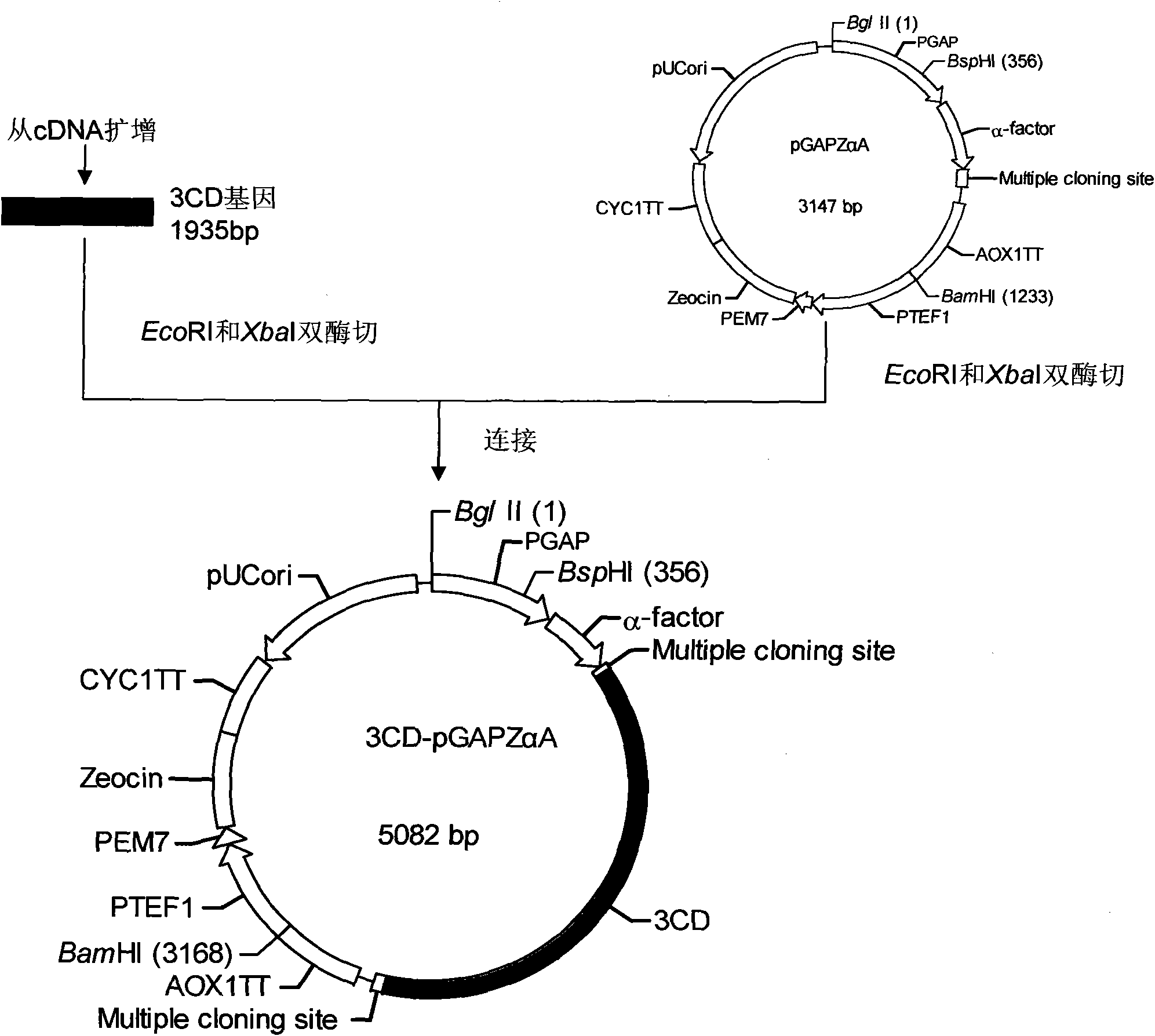
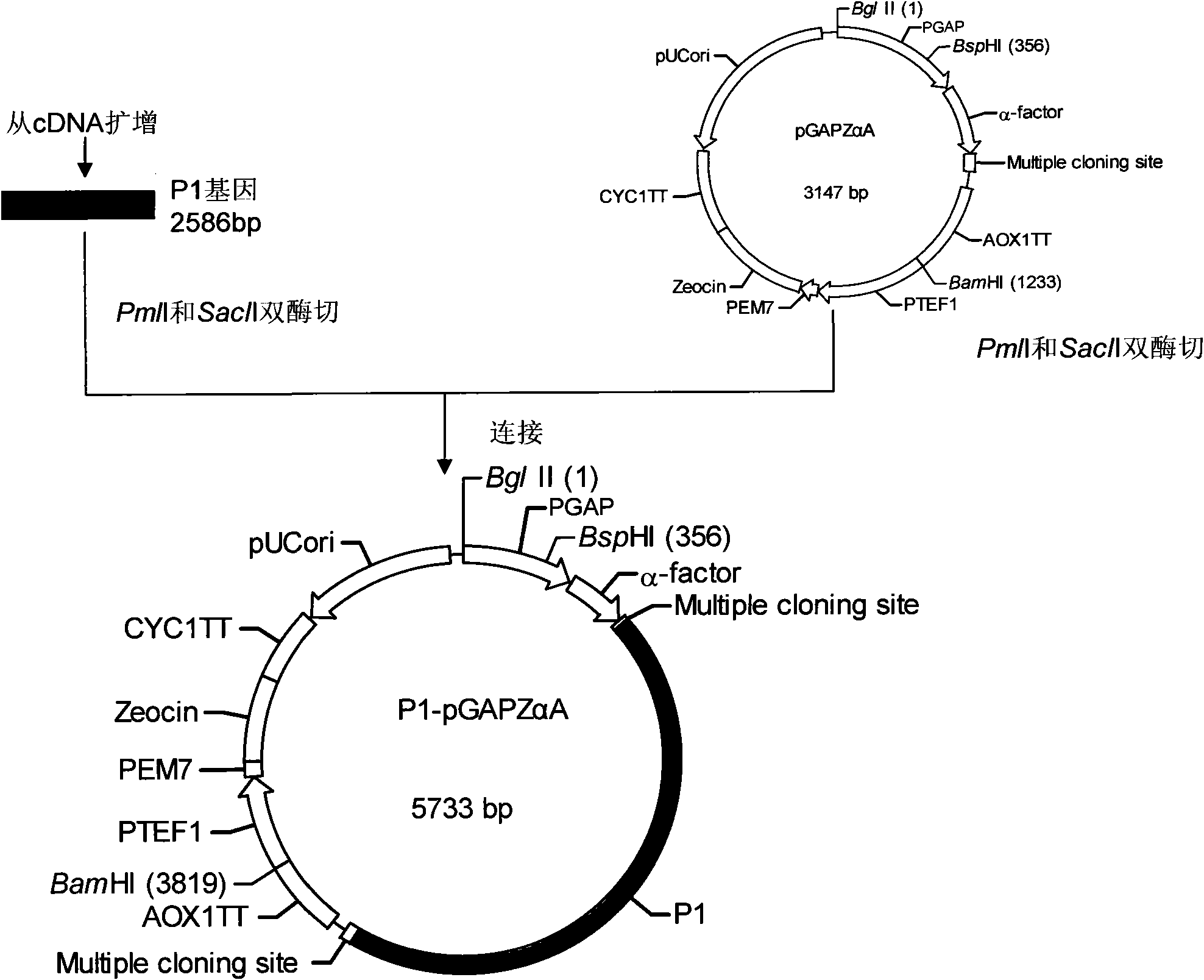
[0037] 1. P1 gene, 3CD gene cloning
[0038] 1. First-strand cDNA preparation
[0039] The EV71 viral RNA was reverse-transcribed into cDNA with the Shanghai Sangon Recombinant Avian Myeloblastosis Virus Reverse Transcriptase (AMV) First-Strand cDNA Reverse Transcription Kit.
[0040] 2. Primer Design
[0041] The P1 gene and 3CD gene primers were designed with the help of Primer Premier 5.0 software, and were synthesized by Shanghai Sangong. The underline is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com