Electrolytic foil and producing method thereof
A technology of electrolytic copper foil and electrolyte, applied in electrolytic process, electroforming, etc., can solve the problems of increased process and ineffective use of tin plating layer, etc., and achieve the effect of high industrial applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Prepared by 100g / L of sulfuric acid (H 2 SO 4 ) and 280g / L copper sulfate pentahydrate (CuSO 4 ·5H 2 O) formed sulfuric acid-copper sulfate aqueous solution (hereinafter referred to as the electrolyte solution "basic electrolyte solution").
[0061] As additives, polyglycerin (trade name: polyglycerol; product code: PGL-X; manufactured by Daicel Chemical Industry Co., Ltd.), 3-mercapto-1-propanesulfonate sodium, N,N-diethylthiourea (commercial Name: Sansera-EUR; manufactured by Sanshin Chemical Industry) and hydrochloric acid were added to the basic electrolyte solution, and relative to the basic electrolyte solution, the concentration was adjusted so that polyglycerin was 30 mg / L, and sodium 3-mercapto-1-propanesulfonate was 5 mg / L, 1.5 mg / L of N,N-diethylthiourea, and 35 mg / L of chloride ion (see Table 1 described later).
[0062] The electrolytic solution containing the above-mentioned additives is supplied between the insoluble anode made of titanium (coated wit...
Embodiment 2~5、 comparative example 1~4
[0086] In addition to changing the type of additive and the concentration relative to the basic electrolyte, the electrolytic current density, and the temperature of the electrolyte to the data shown in Table 1 described later, the thickness of 18 μm was obtained under the same conditions as in Example 1. Electrolytic copper foil. And each evaluation test was performed similarly to Example 1 about the obtained unprocessed electrolytic copper foil. The results are shown in Table 2 and Table 3 described later.
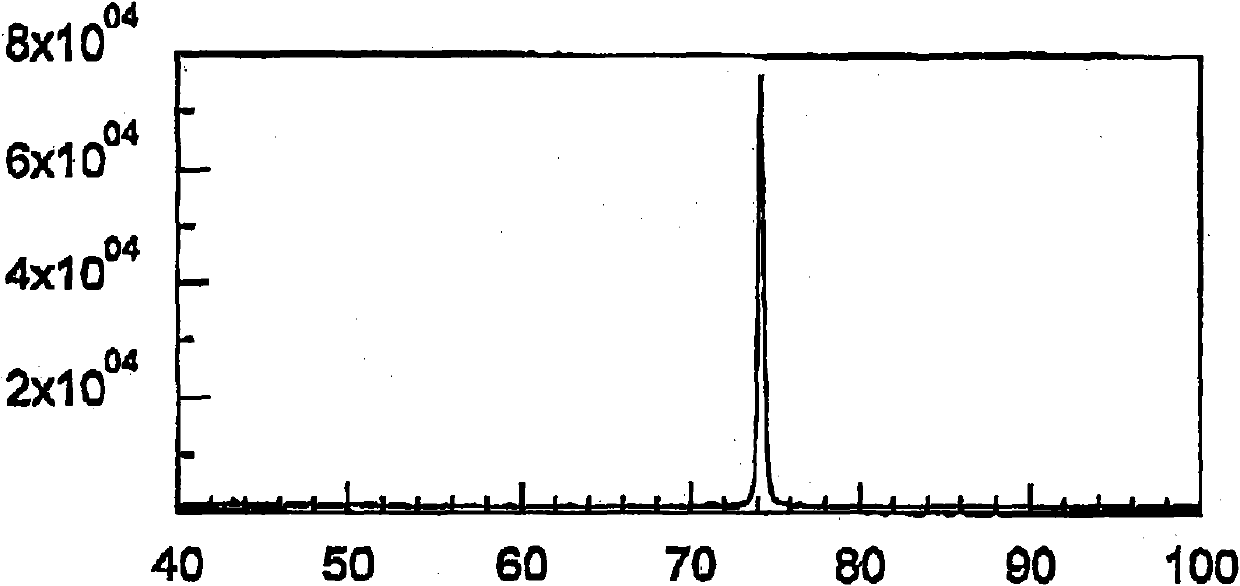
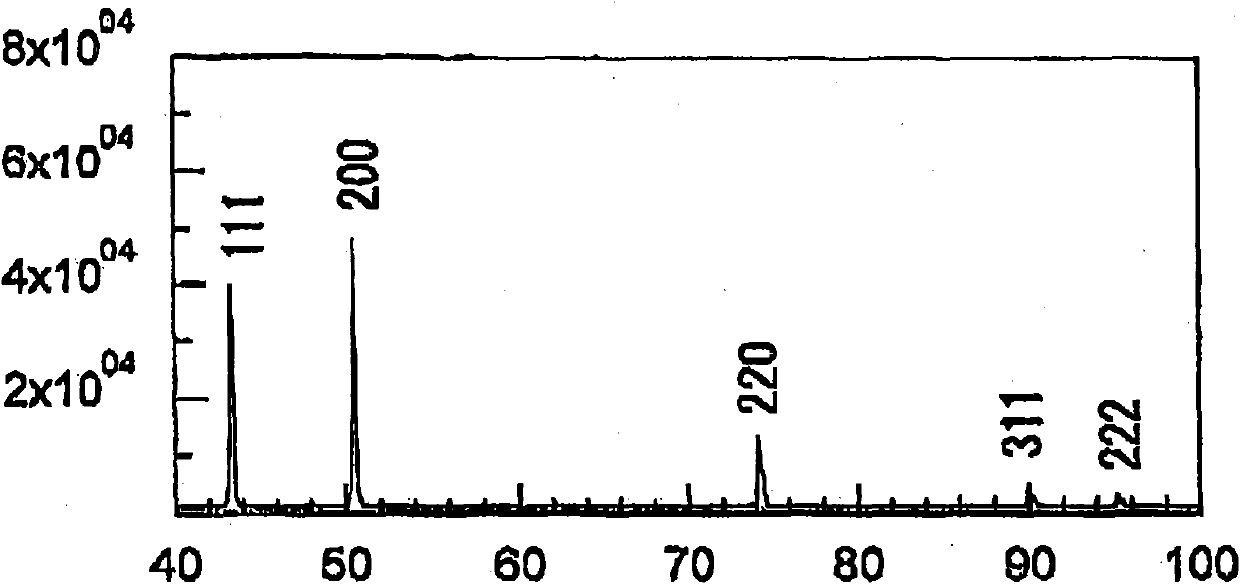
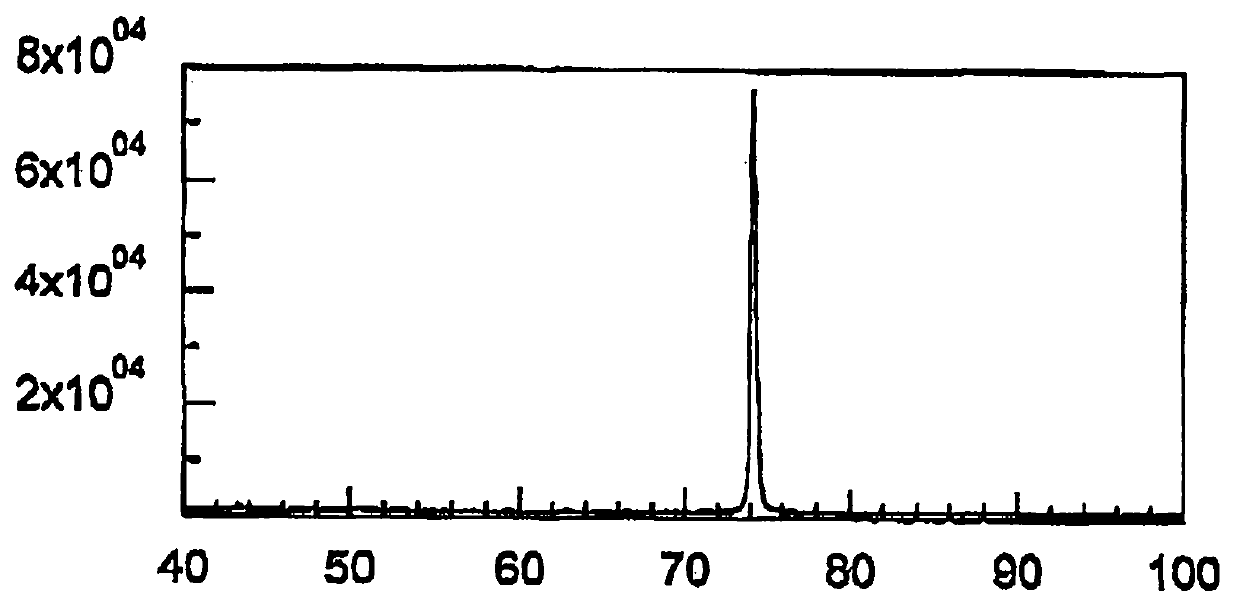
[0087] figure 1 An X-ray diffraction pattern showing the rough side of the untreated electrodeposited copper foil obtained in Example 1, figure 2 An X-ray diffraction pattern on the rough side of the untreated electrodeposited copper foil obtained in Comparative Example 1 is shown.
[0088] Depend on figure 1 It was observed that the intensity of the 220 diffraction line was strong, and thus it was found that a copper foil having a crystal structure with a strong {1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com