Isatin derivatives for use as in vivo imaging agents
A compound and composition technology, applied in the field of new isatin 5-sulfonamide derivatives, can solve the problems of increased non-specific binding, poor image contrast, high lipophilicity, etc., and achieve high metabolic stability, accurate visualization and quantification Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0175] Synthesis of Example 1-Compounds
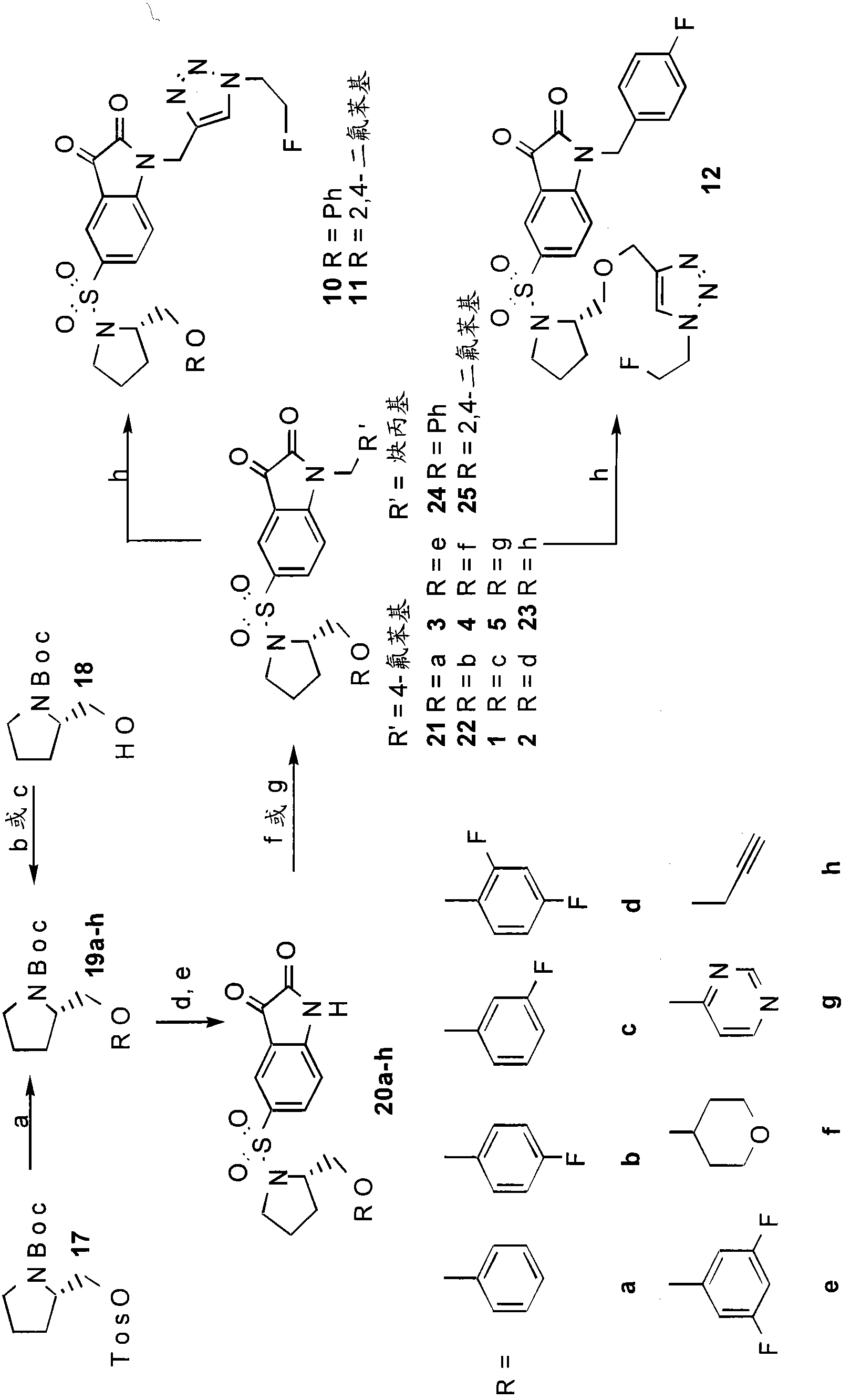
[0176] A target compound library was created using ((S)-1-(4-fluorobenzyl)-5-(2-phenoxymethyl-pyrrolidine-1-sulfonyl)isatin) as a lead compound. Modifications are made to the left side ether moiety and the N-1 position. A fluoro group was incorporated into the left-hand phenyl ether group, and heterocycle and alkyne tolerance at this position was investigated. The tolerance of the 1,2,3 triazole group at the N-1 position was also investigated. The target compound was synthesized by condensation of a functionalized pyrrolidine with 5-chlorosulfonyl isatin followed by alkylation of the isatin nitrogen using potassium carbonate / DMF, as figure 1 shown. All necessary starting materials are commercially available or as in Lee, D. et al. 8 , Chu, W. et al. and Kopka, K. et al. 13 prepared in.
[0177] Reaction of commercially available phenol as well as 4-hydroxytetrahydropyran with tosylate 17 provided pyrrolidines 19a-f in relativel...
Embodiment 2
[0225] Example 2 - Measurement of lipophilicity and affinity for caspases of target compounds
[0226] Affinity of target compounds for caspases New fluoroisatins 1-5 and 10-12, and existing isatin derivatives and intermediate compounds 22 and 23 for different activated caspases The affinities of 1, 3, 6, 7, and 8 were all measured by fluorescent in vitro caspase inhibition assays in collaboration with Kopka and his colleagues 13 Similar to what was stated. Inhibition of recombinant human caspases was assessed by measuring the accumulation of the fluorescent product 7-amino-4-methylcoumarin (7-AMC).
[0227] Recombinant human caspase-1, -3, -6, -7 and -8 and their peptide-specific substrates were purchased from Biomol International, UK. Inhibition of recombinant caspases by non-radioactive isatin was evaluated by fluorescence analysis measuring the accumulation of the fluorescent product 7-amino-4-methylcoumarin (7-AMC). All assays were performed in 96-well plates at a volu...
Embodiment 3-
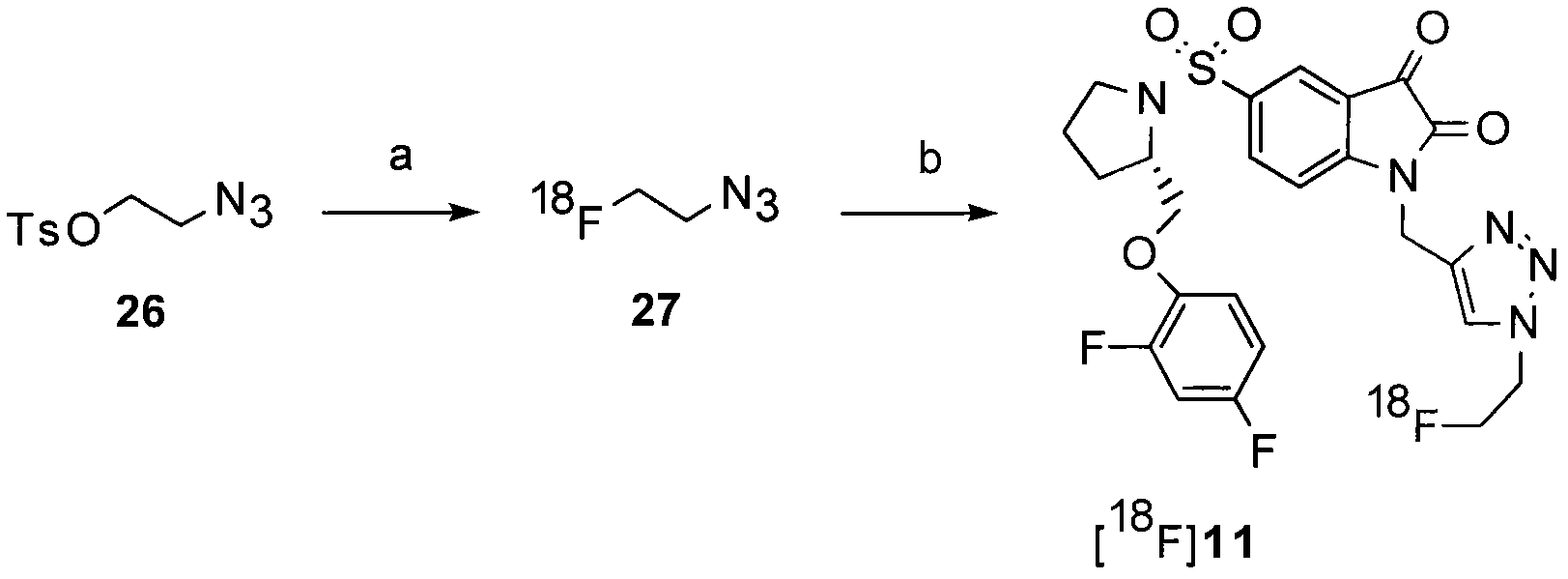
[0240] Embodiment 3-[ 18 Synthesis of F]11
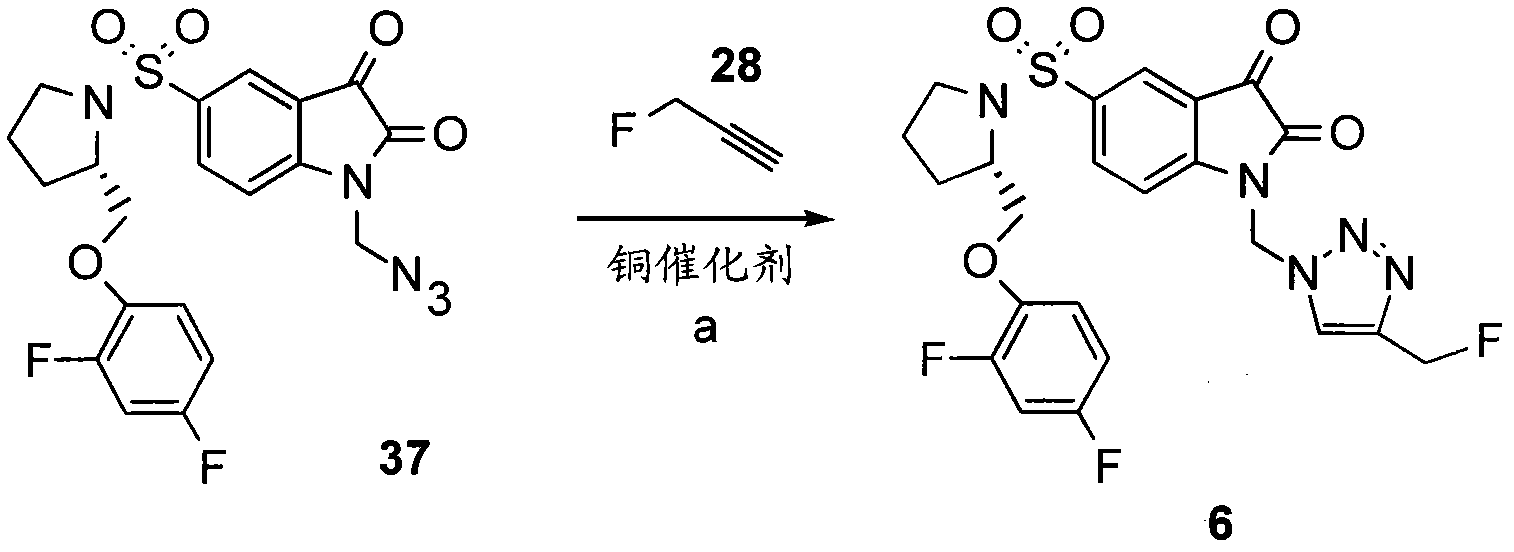
[0241] Preferably, the compound [ 18 F]11 is used [ 18 F] Fluoroethyl azide (compound 27) "click-label" (S)-1-(2-propynyl)-5-(2-(2,4-difluorophenoxymethyl)-pyrrolidine -1-sulfonyl) isatin (compound 25) produces 15,16 . Triazole (S)-1-((1-(2-fluoroethyl)-1H-[1,2,3]-triazol-4-yl)methyl)-5-(2(2,4- Difluorophenoxymethyl)-pyrrolidine-1-sulfonyl) isatin (compound 11) is obtained by 2-[ 18 F] fluoroethyl azide (compound 27) and alkyne precursor (S)-1-(2-propynyl)-5-(2-(2,4-difluorophenoxymethyl)-pyrrolidine- 1-sulfonyl)isatin (compound 25) was labeled by copper-catalyzed cycloaddition, as shown in Figure 2(i) and Figure 19 shown in . The same approach can be used to generate non-radiolabeled compound 11, using fluoroethyl azide instead of 2-[ 18 F] Fluoroethyl azide.
[0242] Under a nitrogen atmosphere, a buffer solution (sodium phosphate buffer, pH 6.0, 250 mM) of sodium ascorbate (50 μl, 8.7 mg, 43.2 μmol) was added to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com