Method for preparing nano lithium carbonate
A lithium carbonate and nano technology, applied in the direction of nano technology, nano technology, nano structure manufacturing, etc., to achieve the effect of convenient operation, simple equipment and narrow particle size distribution range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
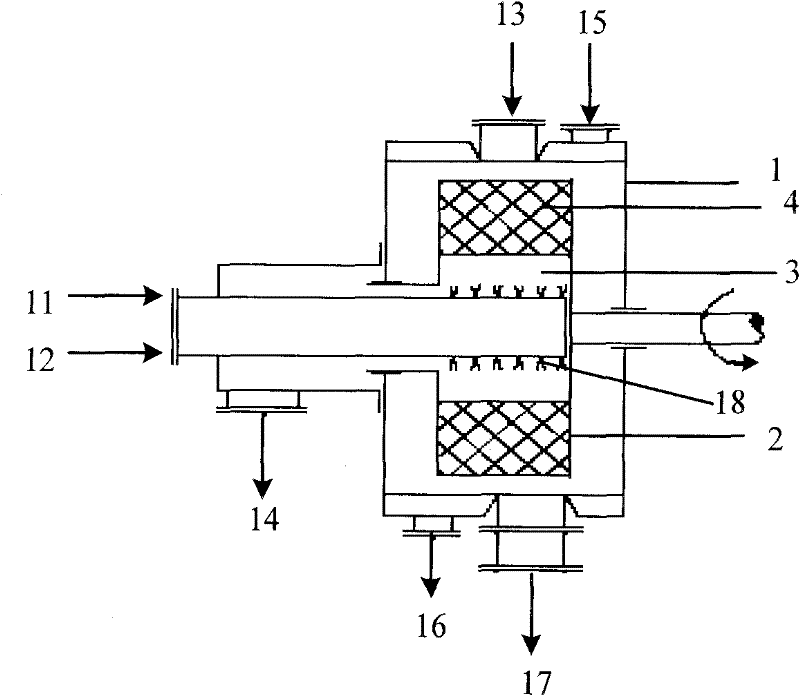
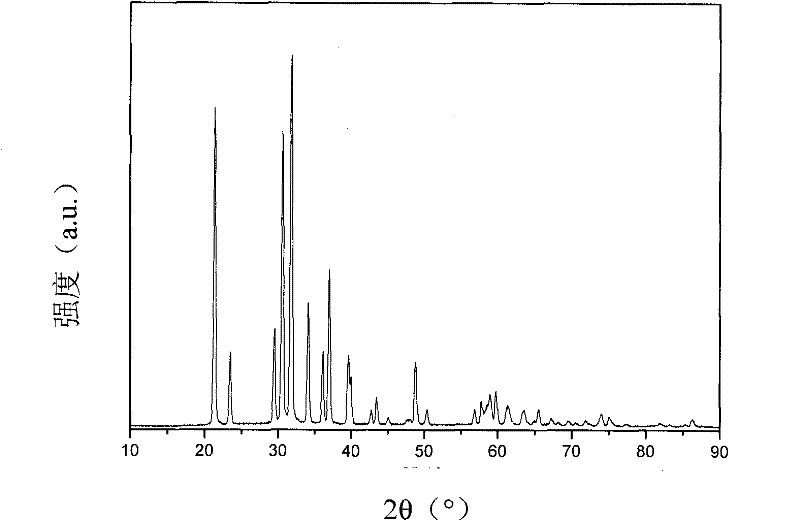
[0030] Adjust the rotating speed of the rotating packed bed to 1000r·min -1 , The 4.0mol·L -1 Lithium hydroxide and 0.1g·L -1 The mixed solution of sodium lauryl sulfate is 0.1L·min -1 The speed is input to the rotating packed bed through the metering pump, and at the same time, the speed is 4.0L·min -1 Enter carbon dioxide gas at a flow rate of 40°C. The reaction temperature is 40°C. The raw material solutions are quickly and fully mixed under the action of large centrifugal force. The reaction crystallizes to produce nano-lithium carbonate particles. The reacted mixture is discharged from the discharge port of the rotating packed bed, and is Dry in an oven at 200°C for 2 hours to obtain nano-scale lithium carbonate with a particle size of 10-30 nm. The X-ray diffraction photos of the prepared nano-lithium carbonate are as follows figure 2 Shown.
Embodiment 2
[0032] Adjust the rotating speed of the rotating packed bed to 1500r·min -1 , Add 2.0mol·L -1 Lithium hydroxide and 0.05g·L -1 The mixed solution of sodium dodecyl sulfonate is 2.0L·min -1 The speed is inputted into the rotating packed bed through the metering pump, and 2.0mol·L -1 The reaction temperature is 60℃. The raw material solution is quickly and fully mixed under the action of large centrifugal force. The reaction crystallizes to generate nano-lithium carbonate particles. The reacted mixture is discharged from the discharge port of the rotating packed bed, and is filtered and washed. Dry in an oven at 300°C for 10 hours to obtain nano-scale lithium carbonate with a particle size of 50-80 nm.
Embodiment 3
[0034] Adjust the rotating speed of the rotating packed bed to 3000r·min -1 , 1.5mol·L -1 Lithium chloride and 0.1g·L -1 The mixed solution of polyethylene glycol is 1.0L·min -1 The speed is inputted into the rotating packed bed through the metering pump, and 3.0mol·L -1 The reaction temperature is 30℃. The raw material solution is quickly and fully mixed under the action of large centrifugal force. The reaction crystallizes to generate nano-lithium carbonate particles. The reacted mixture is discharged from the discharge port of the rotating packed bed, filtered and washed Then, it was dried in an oven at 250° C. for 4 hours to obtain nano-scale lithium carbonate with a particle size of 60-80 nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| rotating speed | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com