Aromatic diamine containing imidazolyl and preparation method thereof
A technology containing imidazole-based aromatic diamines and aromatic tetramines, which is applied in organic chemistry and other fields, can solve the problems of few types of imidazole-based diamine-containing monomers and limit the development of imidazole-based polymer varieties, and achieve rich types, Effects of improved performance and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
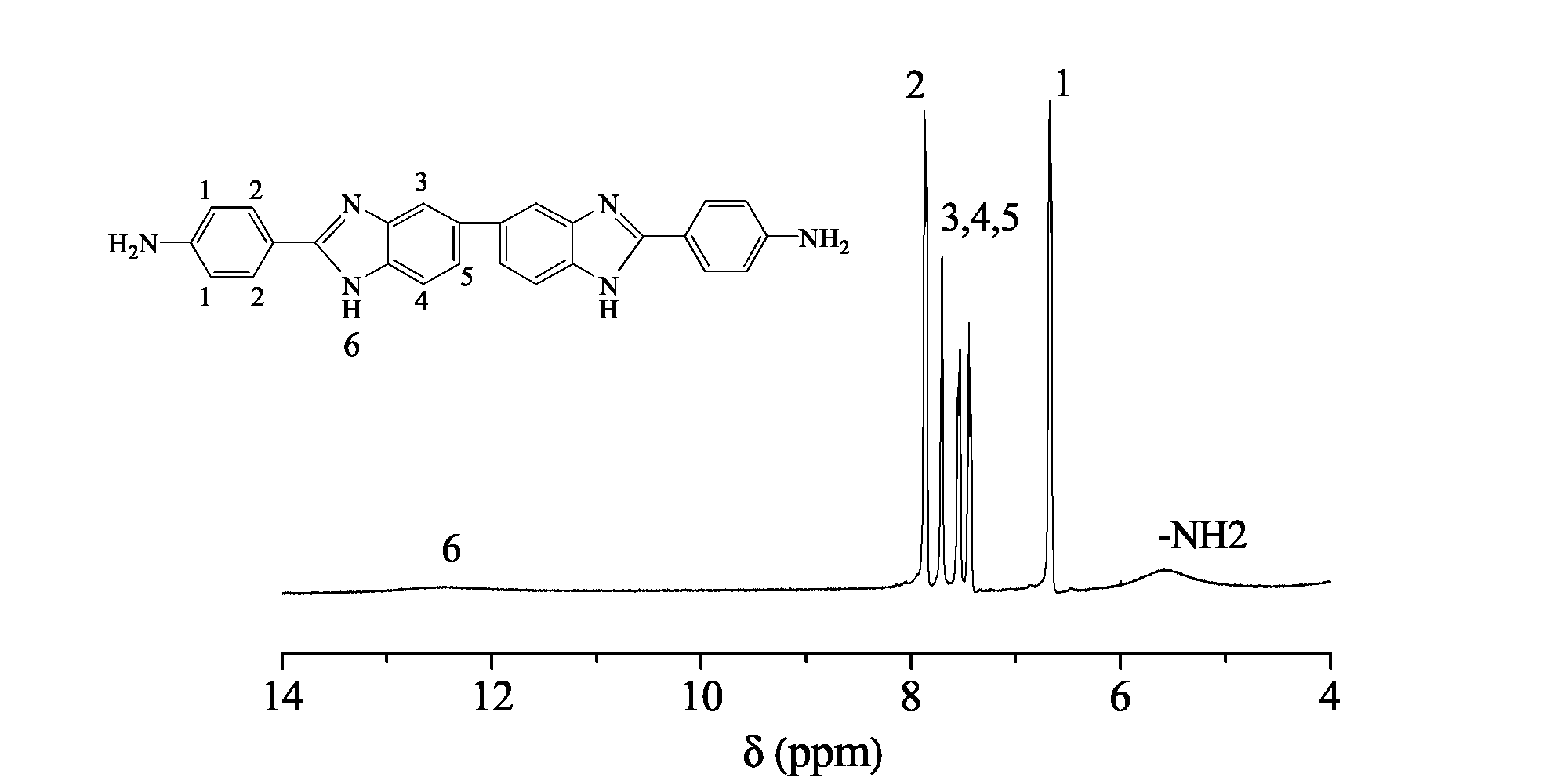
[0030] In a 100mL dry three-necked flask, add 100g of polyphosphoric acid with a mass concentration (calculated as phosphorus pentoxide) of 86%, 5.486g (40mmol) of 4-aminobenzoic acid and 4.2854g (20mmol) of 3,3'- Diaminobenzidine. Under the protection of nitrogen, the temperature was raised to 150 ° C, and after 2 hours of reaction, the temperature was raised to 190 ° C, and the reaction was completed for 20 hours. The product was poured into crushed ice to obtain a light yellow solid, which was fully washed with 0.1M sodium bicarbonate solution. Remove residual phosphoric acid in the solid, then wash with deionized water until neutral, and dry in vacuum at 60°C. The yield is about 85%.
[0031] Spectroscopic characterization of the as-prepared product was carried out. figure 1 It is the nuclear magnetic resonance spectrum of 2,2'-bis(4-aminophenyl)-5,5'-bibenzimidazole (solvent: DMSO-d 6 ), the attribution of each peak is marked in the spectrum respectively: δ=12.4ppm (b,...
Embodiment 2
[0034] In a 100mL dry three-necked flask, add 100g of polyphosphoric acid with a mass concentration (calculated as phosphorus pentoxide) of 86%, 10.972g (80mmol) of 4-aminobenzoic acid and 4.2854g (20mmol) of 3,3'- Diaminobenzidine. Under the protection of nitrogen, the temperature was raised to 150°C, and after 2 hours of reaction, the temperature was raised to 190°C, and the reaction was completed for 20 hours. The product was poured into crushed ice to obtain a light yellow solid, which was fully washed with 0.1M sodium hydroxide solution. Remove residual phosphoric acid in the solid, then wash with deionized water until neutral, and dry in vacuum at 60°C. The yield is about 87%.
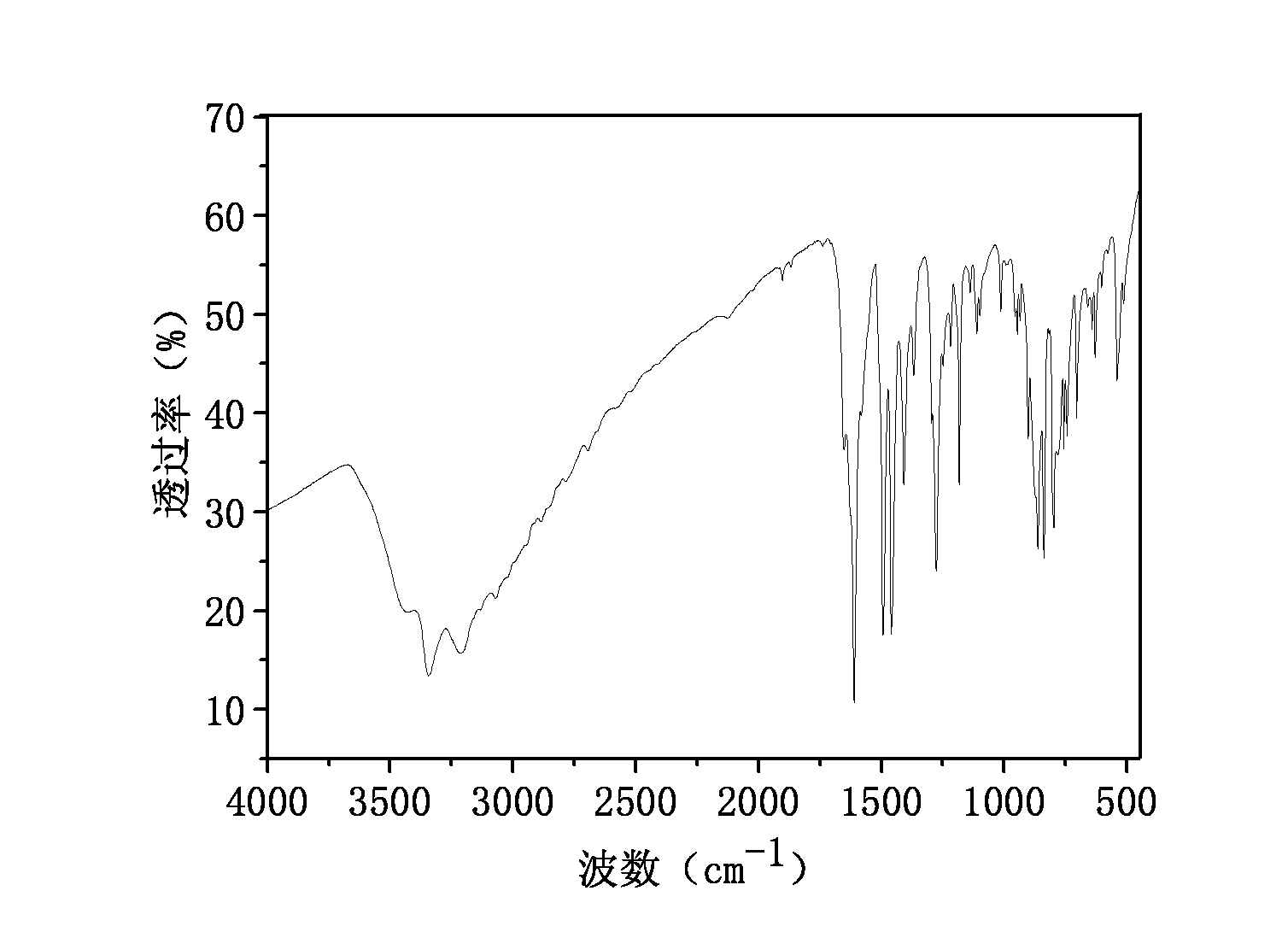
[0035] The prepared product has been carried out spectroscopic characterization, and the nuclear magnetic resonance spectrogram and infrared spectrum that record are identical with embodiment 1 ( figure 1 with 2 ).
Embodiment 3
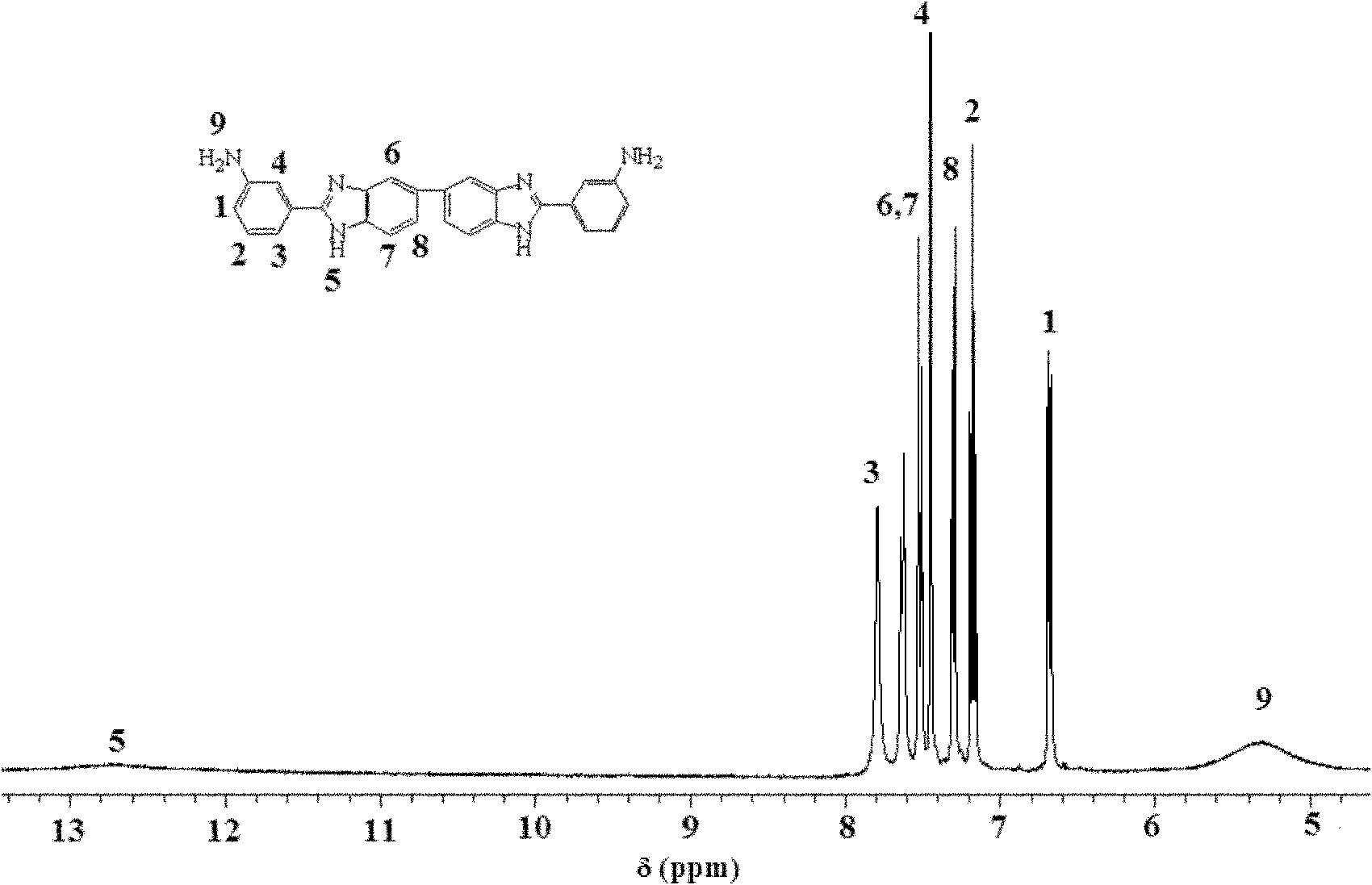
[0037]In a 100mL dry three-necked flask, add 100g of polyphosphoric acid with a mass concentration (calculated as phosphorus pentoxide) of 86%, 5.486g (40mmol) of 3-aminobenzoic acid and 4.2854g (20mmol) of 3,3'- Diaminobenzidine. Under the protection of nitrogen, the temperature was raised to 140°C, and after 3 hours of reaction, the temperature was raised to 190°C, and the reaction was completed for 20 hours. After the reaction was completed, the product was poured into crushed ice to obtain a light yellow solid, which was placed in 1M ammonia water, stirred and washed thoroughly. Remove residual phosphoric acid in the solid, then wash with deionized water until neutral, and dry in vacuum at 60°C. The yield is about 80%.
[0038] Spectroscopic characterization of the as-prepared product was carried out. image 3 It is the nuclear magnetic resonance spectrum of 2,2'-bis(3-aminophenyl)-5,5'-bibenzimidazole (solvent: DMSO-d 6 ), the attribution of each peak is marked in the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com