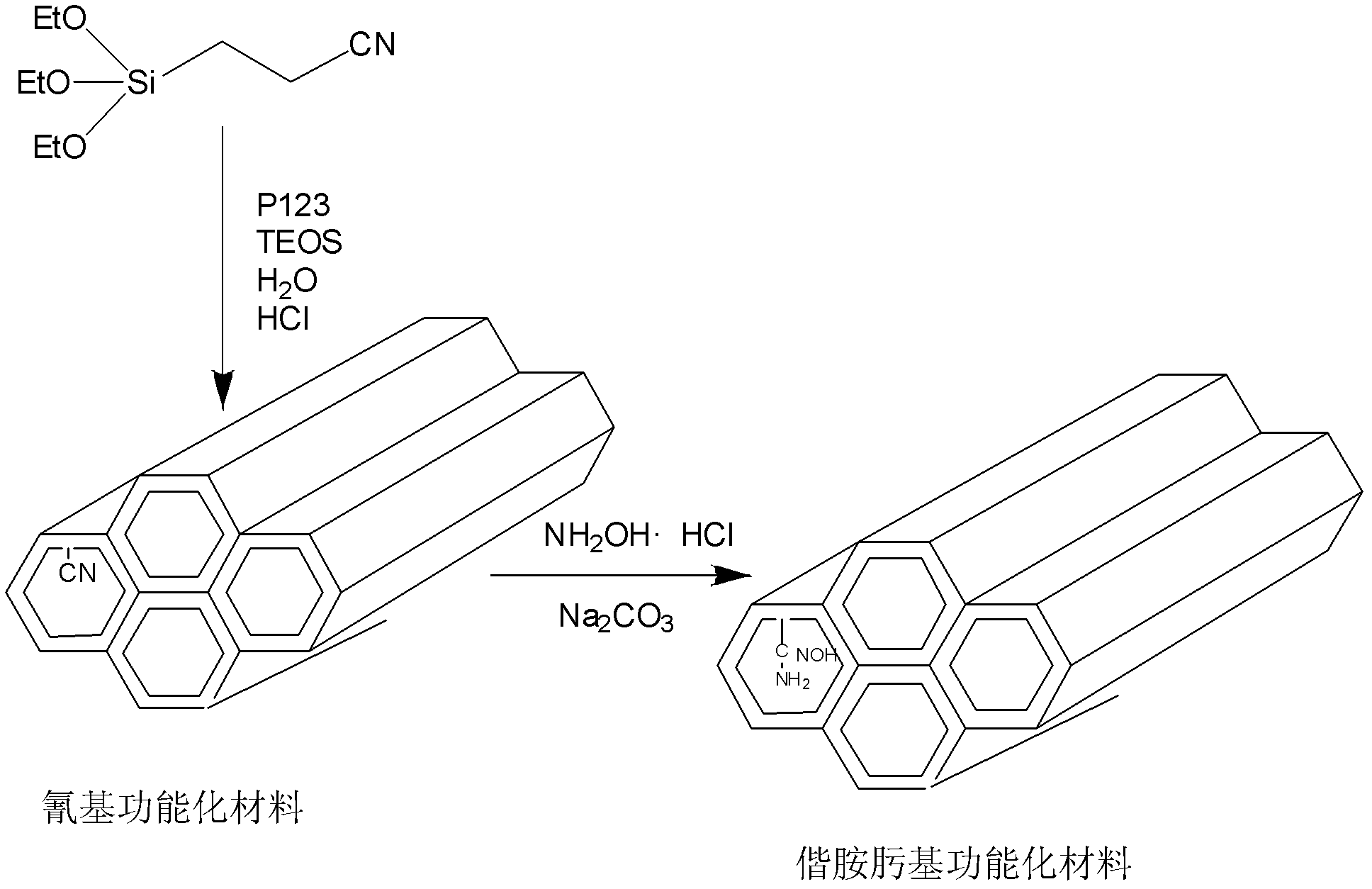
Amidoxime group uranium extraction sorbent and preparation method thereof
An amidoxime-based, adsorbent technology, applied in chemical instruments and methods, uranium compounds, inorganic chemistry, etc., can solve the problems of shortening the service life of materials, poor hydrophilicity, slow adsorption speed, etc. Water-based enhanced, highly selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1) Dissolve 1.0 g of nonionic surfactant P123 in 37.5 mL of 1.6 mol / L hydrochloric acid solution, and stir at room temperature until clear. Then add mixed silicon esters (TEOS and CTES), stir at 40°C for 22h, transfer the reaction liquid to a reaction kettle with polytetrafluoroethylene, leave it to age at 70°C for 24h, then cool, filter and dry. The molar ratio of each raw material used in the experiment is P123:H 2 O:HCl:CTES:TEOS=0.017:193.3:5.9:0.2:0.8.
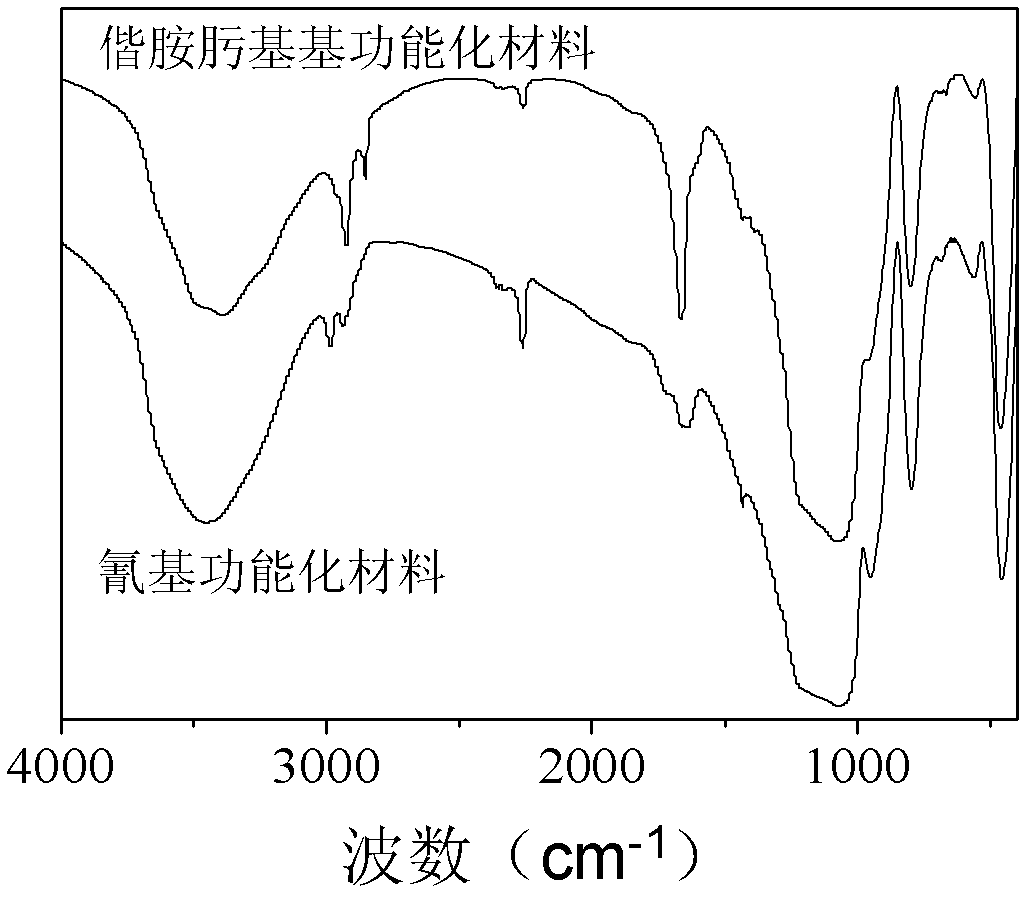
[0027] 2) Disperse 1.0 g of the dried synthetic material in 1) in 150 mL of absolute ethanol containing 2.0 g of concentrated hydrochloric acid, stir at 50°C for 6 hours, then filter, wash with absolute ethanol, and dry to obtain the cyano-functionalized organic - Inorganic hybrid porous materials;
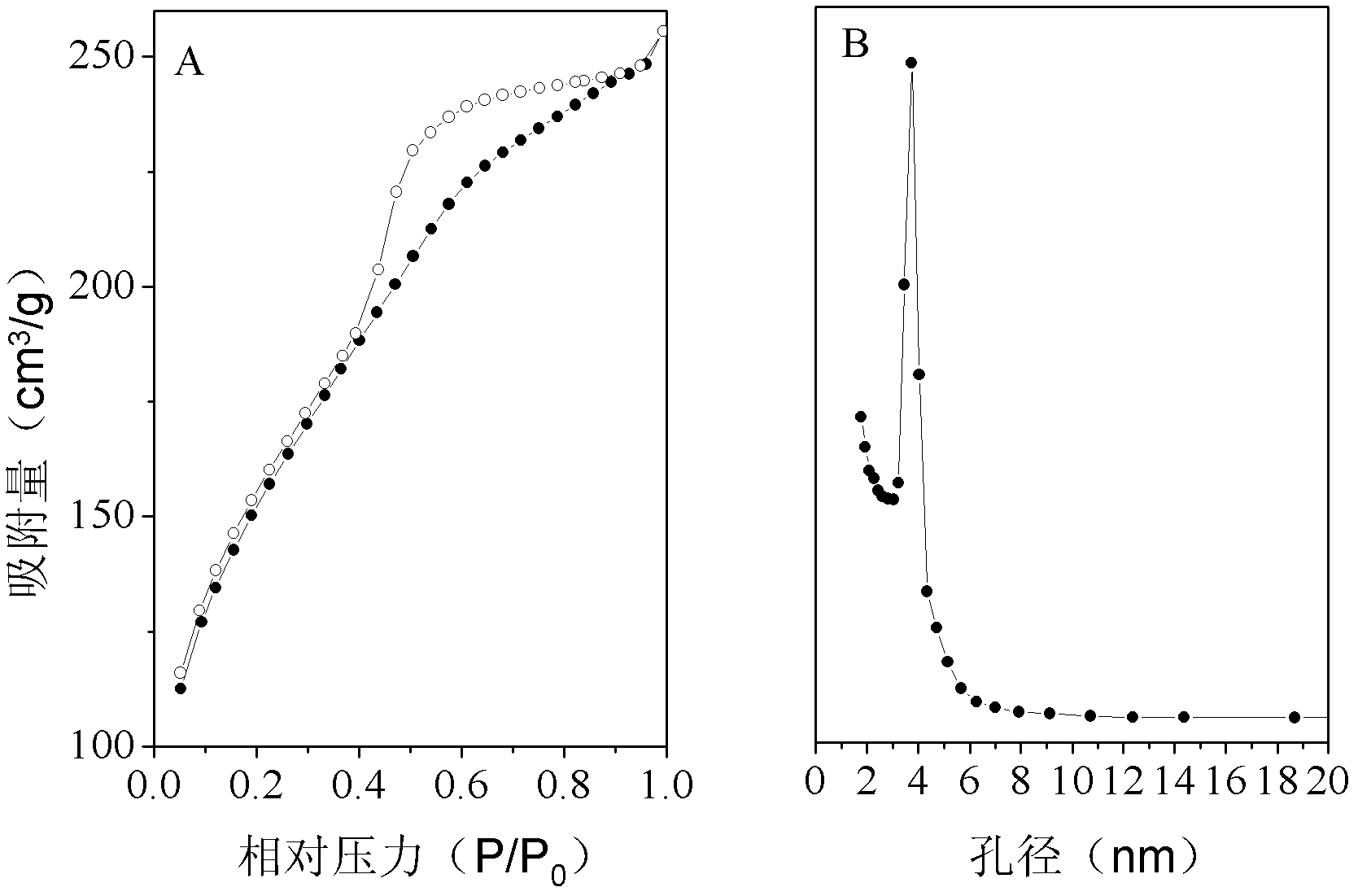
[0028] From figure 2 It can be seen that the adsorption equilibrium isotherm of the material is type IV, indicating that the material has mesopores. The material has a specific surface area of 533m 2 / g, the po...
Embodiment 2
[0033] 1) Dissolve 1.0 g of nonionic surfactant P123 in 37.5 mL of 1.6 mol / L hydrochloric acid solution, and stir at room temperature until clear. Then add mixed silicon esters (TEOS and CTES), stir at 80°C for 10h, transfer the reaction liquid to a reaction kettle with polytetrafluoroethylene, leave it to age at 60°C for 24h, then cool, filter and dry. The molar ratio of each raw material used in the experiment is P123:H 2 O:HCl:CTES:TEOS=0.017:193.3:5.9:0.4:0.6.
[0034] 2) Disperse 1.0 g of the dried synthetic material in 1) in 150 mL of absolute ethanol containing 2.0 g of concentrated hydrochloric acid, stir at 50°C for 6 hours, then filter, wash with absolute ethanol, and dry to obtain the cyano-functionalized organic - Inorganic hybrid porous material; the specific surface area of the material is 312m 2 / g, the pore diameter is 2.68nm, and the cyano group content is 4.30mmol / g.
[0035] 3) In a 100mL three-necked flask, 0.76g of nitrile functionalized material (NH ...
Embodiment 3
[0037] Pipette 50 mL of U(VI) solution with an initial concentration of 100 μg / mL, adjust the pH of the solution to ≈6, add 0.01 g of the adsorbent prepared in Example 1, shake and balance at 30 ° C for 90 min, filter, and use Arsenazo III spectrophotometry The concentration of U(VI) in the solution before and after adsorption was analyzed by the method, and the amount of uranium adsorbed was calculated.
[0038] From Figure 5 It can be seen that the adsorption rate of U(VI) on the material is very fast. This is because the adsorbent used has a large specific surface area, a mesoporous pore structure, and strong hydrophilicity, which reduces the adsorption capacity of uranyl ions. The mass transfer resistance makes it easy for uranyl ions to contact with the amidoxime adsorption sites, thereby increasing the adsorption rate. The equilibrium adsorption capacity is as high as 310mg / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com