Dispersion tablets of traditional Chinese medicine composition
A technology for dispersible tablets and compositions, which can be used in drug combinations, plant/algae/fungus/moss ingredients, pharmaceutical formulations, etc., and can solve problems such as adverse reactions, long disintegration time, and large dosage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0033] Experimental example 1 Prescription Screening Experiment
[0034] 1. Instruments and raw materials
[0035] Tablet press: (Model: DP30);
[0036] Disintegration instrument: (model: ZB6);
[0037] Balance: (Model: FA1004, manufacturer: Shanghai Balance Instrument Factory).
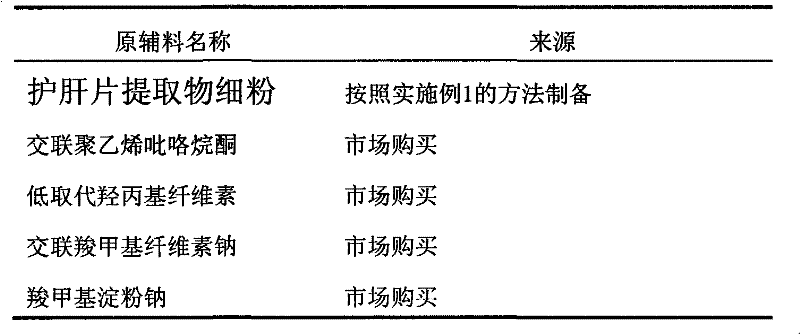
[0038] Table 1 Sources of raw and auxiliary materials
[0039]
[0040]
[0041] 2 methods:
[0042] (1), disintegrant selection:
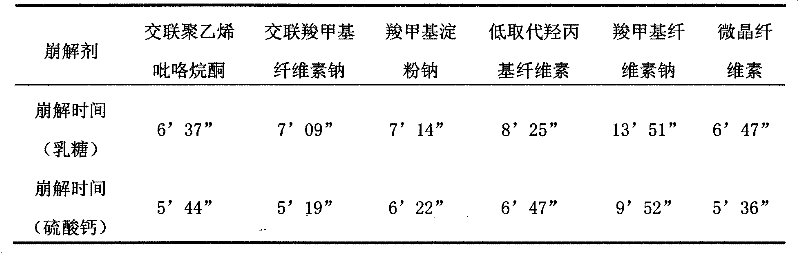
[0043] In the case of constant drug powder and filler in this prescription, the drug powder and disintegrant were mixed according to the ratio of 1:1, compressed into tablets, and the disintegration time was used as the investigation index. The results are shown in Table 2:
[0044] Table 2 Screening of disintegrants
[0045]
[0046] The results showed that the disintegration effects of cross-linked polyvinylpyrrolidone, cross-linked sodium carboxymethyl cellulose, sodium carboxymethyl starch, low-substituted hydroxypropyl cellulose and microcrystalline ...
experiment example 2
[0067]Experimental Example 2 Bioequivalence Experiment
[0068] Dispersible tablet of the present invention has carried out human body bioequivalence test result with certain listed common tablet and shows: the InC of two kinds of preparations max 、InAUC 0→24 The three-factor analysis of variance showed that the InC of the two preparations max 、InAUC 0 →24 There are significant differences. The average maximum blood drug concentration of the tested preparations was higher than that of ordinary tablets. T of the two formulations max Using non-parametric test, the test results have significant difference, the peak time T of the tested preparation max Significantly faster than conventional tablets; InC max 、InAUC 0→24 The double one-sided t test showed that the dispersible tablet was superior to the ordinary tablet.
[0069] The following embodiments can achieve the effects of the above experimental examples.
Embodiment 1
[0071] Liver extract fine powder 60g
[0072] Sodium carboxymethyl starch 30g
[0073] Cross-linked polyvinylpyrrolidone 45g
[0074] Croscarmellose Sodium 20g
[0075] Microcrystalline Cellulose 40g
[0076] Low-substituted hydroxypropyl cellulose 20g
[0078] Lactose 160g
[0079]
[0080] Makes 1000 pieces
[0081] Preparation:
[0082] Preparation of liver-protecting tablet extract powder: Bupleurum 313g, capillary root 313g, isatidis 313g, schisandra 168g, pig gall powder 20g, mung bean 128g; Bupleurum, capillary, isatidis, and mung bean (crushed) were decocted twice, each time for 2 hours, Filtrate, combine the filtrates, and concentrate under reduced pressure to obtain a clear cream A with a relative density of 1.30 (80°C); grind Schisandra chinensis into coarse powder and soak pig bile powder in 90% ethanol for 4 hours, reflux extraction for 1 hour, and dynamic heat reflux for 6 hours , rec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com