Cattle Nramp1 macrophage specificity expression vector as well as construction method and application thereof
An expression vector and construction method technology, applied in the field of genetic engineering, can solve problems such as troubles in the prevention and control of intracellular parasitic bacteria, and achieve the effects of reducing adverse effects and increasing expression levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
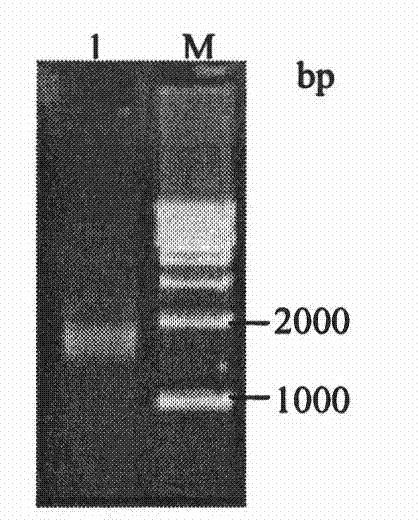
[0044] Example 1 Isolation, amplification and sequencing of Nramp1 gene fragments
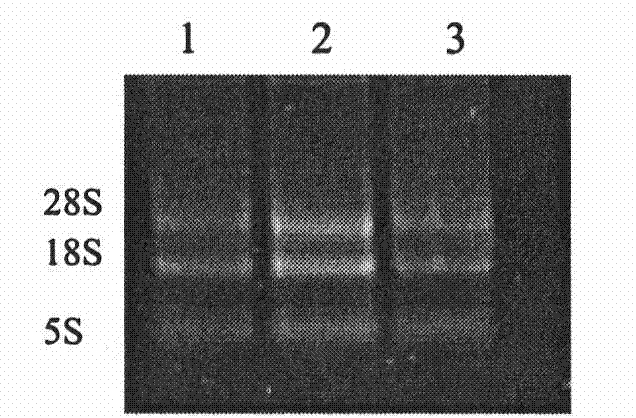
[0045] 1.1 Extraction of total RNA
[0046] The jugular vein blood of Qinchuan cattle (Shaanxi Yangling Keyuan Bioengineering Co., Ltd.) was aseptically collected, and red blood cell lysate (Triis-NH 4 Cl), mix at 4°C for 4 to 5 minutes (during which repeated gentle inversion and mixing) to remove red blood cells, wait until the red blood cells are completely broken, centrifuge at 1000r / min for 5 minutes to obtain white blood cell pellet, wash with PBS 2 to 3 times, and centrifuge to precipitate. Add an appropriate amount of TRIzol reagent, let it stand at room temperature for 5 minutes, add 1 / 5 TRIzol reagent amount of chloroform, shake and mix, let it stand at room temperature for 5 minutes, centrifuge at 12000r / min at 4°C for 10 minutes, absorb the supernatant and transfer it to a new centrifuge tube, add an equal volume of Isopropanol, let stand at room temperature for 10min, centrifuge at...
Embodiment 2
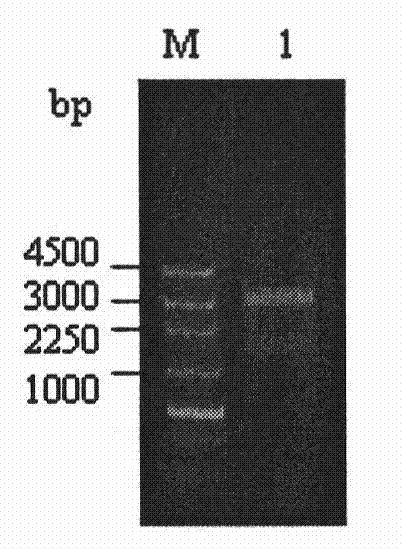
[0065] Example 2 Construction and Identification of Nramp1 Eukaryotic Expression Vector Recombinant Plasmid pIRES2-N
[0066] The pMD18-N4 and pIRES2-EGFP vectors were digested with BamH I and Sal I respectively, and the target gene fragments recovered from the gel were ligated with the corresponding vector fragments with T4 DNA ligase overnight at 16°C, and the ligated products were transformed into DH5a competent bacteria, and after 16 hours Single clones were picked and shaken, amplified, and recombinant plasmids were extracted for enzyme digestion identification, and the identified correct recombinant plasmids were named pIRES2-N( Figure 4 ).
Embodiment 3
[0067] Example 3 Construction and identification of phagocyte-specific expression vector pIRES2-PN
[0068] 3.1 Blood genome extraction
[0069] (1) Take 200 uL of peripheral blood samples from Holstein cows (Shaanxi Yangling Keyuan Bioengineering Co., Ltd.), add 20 uL of proteinase K solution, and mix well. Add 200uL of buffer GB again, mix thoroughly by inversion, and place at 70°C for 10 minutes. After the solution becomes clear, centrifuge immediately to remove the water droplets on the inner wall of the tube cap.
[0070] (2) Add 200uL absolute ethanol, shake fully for 15s, and centrifuge briefly.
[0071] (3) Add the solution and flocculent precipitate obtained in the previous step into the adsorption column CB3, centrifuge at 13400×g for 30 s, discard the waste liquid, and put the adsorption column CB3 back into the collection tube.
[0072] (4) Add 500uL buffer GD to the adsorption column CB3, centrifuge at 13400×g for 30s, discard the waste liquid, and put the adsor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com