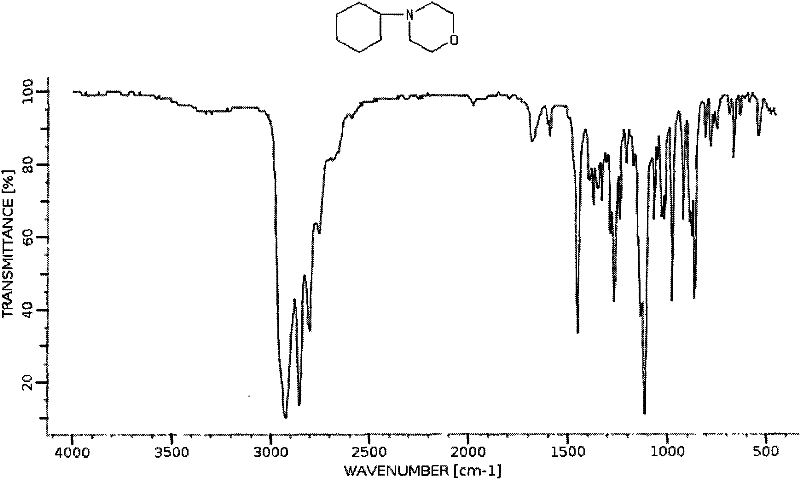
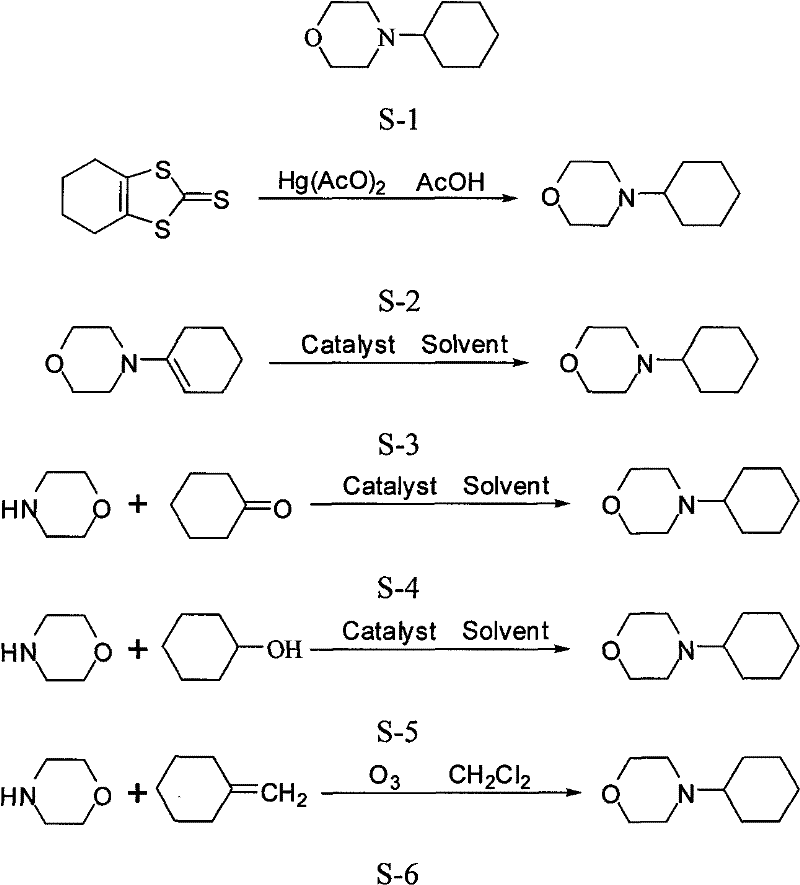
Method for synthesizing 4-cyclohexyl morpholine
A technology of cyclohexylmorpholine and a synthesis method, which is applied in the synthesis field of 4-cyclohexylmorpholine, can solve the problems of difficulty in realizing industrialized production, large equipment investment and high production cost, and achieves low price, high yield and utilization of raw materials. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
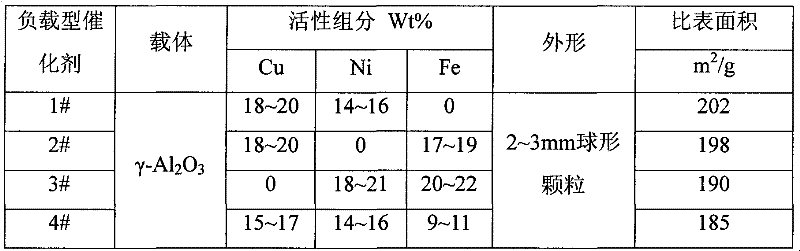
[0029] (1) Preparation of copper-nickel catalyst
[0030] a), the commercially available γ-Al 2 o 3 Roast at 450°C for 3h and 750°C for 5h before use;
[0031] b) Dissolve 41.2g of copper nitrate and 34.2g of solid nickel nitrate (excluding crystal water) in 50ml of distilled water, and impregnate the alumina that has undergone high-temperature pretreatment at the ratio of 1.2ml of solution / 1g of alumina. In the solution, impregnate 36hr; Filter, obtain filtrate and catalyst;
[0032] c), drying the catalyst obtained by filtration at 60°C for 2h, then putting it into a muffle furnace and roasting at 80°C, 150°C, and 280°C for 3h respectively, and then cooling down naturally;
[0033] d), the catalyst obtained after the above-mentioned cooling is put into the filtrate obtained in step b) and soaked for 24h, and filtered;
[0034] e) Dry the catalyst obtained in step d) at 80°C for 2h, then put it into a muffle furnace and roast at 150°C for 2h, then at 450°C for 4h, then co...
Embodiment 1
[0041] Embodiment 1, a kind of production method of 4-cyclohexylmorpholine, carry out following steps successively:
[0042] 1), prepare copper-nickel catalyst.
[0043] 2) Load the prepared supported catalyst into a fixed-bed reactor, heat at 200°C, and activate by purging with hydrogen until the activation is complete, that is, no condensed water is produced in the lower part of the reactor.
[0044] 3) Mix 42.5g (0.4mol) of diethylene glycol with 73.8g (0.8mol) of cyclohexylamine and place it in a mixing tank. The mixed solution is vaporized by the top preheater of the fixed bed and enters the catalyst bed together with hydrogen Carry out the hydroamination reaction, the volume space velocity of the raw material solution is 0.2h -1, adjust the hydrogen pressure so that the reactor pressure is 0.8Mpa, the catalyst bed temperature is controlled at 220°C, the product after the reaction is collected in liquid form after condensation, and analyzed and separated after enough pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com