DNA (deoxyribonucleic acid) level-based highly pathogenic blue-eared pig disease JEV (Japanese encephalitis virus) replicon vaccine and application thereof
A porcine blue-ear disease virus, highly pathogenic technology, applied in the field of genetic engineering, can solve the safety hazards of host genome recombination with a large vaccination dose, and achieve the effect of high antibody and strong lymphocyte proliferation response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Construction of Replicon Vaccine for Porcine Highly Pathogenic PRRS
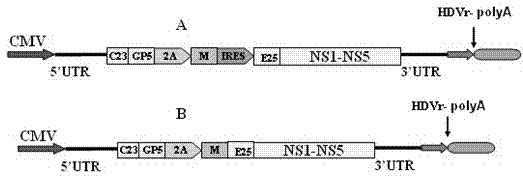
[0027] 1. The design of constructing a replicon vaccine for porcine highly pathogenic PRRS (as shown in the structure diagram figure 1 )
[0028] Through self-designed primers (shown in SEQ ID NO: 19-29), using the reverse transcription product of HP PRRS gene RNA as a template, primers GP5F and GP5R-FMDV2A-R can amplify the complete coding region of the GP5 gene and The first 45 bases of the 2A sequence, the result is a specific fragment of 648bp in size; the primers MF-FMDVF2A-F and MR-SalI can amplify the last 45 bases of the 2A sequence and the complete coding region of the M gene , 570bp was amplified. In addition, the primers GP5F and MR-SalI amplified a G-2A-M fragment of approximately 1,200 bp by fusion PCR. At the same time, G-2A-M fragment was used as template, GP5F and MR were used as primers to amplify G-2A-MR fragment of about 1,200 bp, and then IRES1F and IRES-588R were used as ...
Embodiment 2
[0033] Example 2 Detecting the expression of GP5 protein and M protein by Western-blot with chemiluminescence method
[0034] The plasmids co-expressing GP5 and M proteins of PRRSV (pJEV-REP-G-2A-M and pJEV-REP-G-2A-M-IRES) and eukaryotic expression plasmids (pCAGGS-GM) were respectively transfected into 293T cells, Cells were collected 48 hours after transfection, and 48 hours after recombinant plasmid transfection, cells were harvested, washed twice with PBS, resuspended in appropriate amount of PBS, added 2×SDS loading buffer, and left in boiling water bath for 10 minutes. A 12% SDS-polyacrylamide gel (PAGE) was prepared, spotted at 20μl per well, and electrophoresed at 80V / 0.5h and 120V / 1.5h. After the electrophoresis, the protein was transferred to the nitrocellulose membrane under the condition of 17V / 10min on a semi-dry electrotransfer machine. After the transfer was completed, the membrane was washed with 1×PBS for 5 minutes, and blocked with 5% skim milk at 37°C for 1 ...
Embodiment 3
[0035] Example 3 Indirect immunofluorescence detection of JEV non-structural protein expression
[0036] The JEV replicon plasmids (pJEV-REP-G-2A-M and pJEV-REP-G-2A-M-IRES) co-expressing the GP5 and M proteins of PRRSV and the vector control plasmid (pJEV-REP-IRES) were respectively transformed After 48 hours of infecting 293T cells, it was detected by indirect immunofluorescence that all three JEV replicon recombinant plasmids could express JEV NS1 protein (see Figure 5 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com