Process for synthesizing doripenem lateral chain
A synthesis process and process technology, which is applied in the field of synthesis process of the side chain of doripenem, can solve the problems of complicated route, high price, and cumbersome removal, achieve uniform crystal particles, improve reaction speed, and improve reaction yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
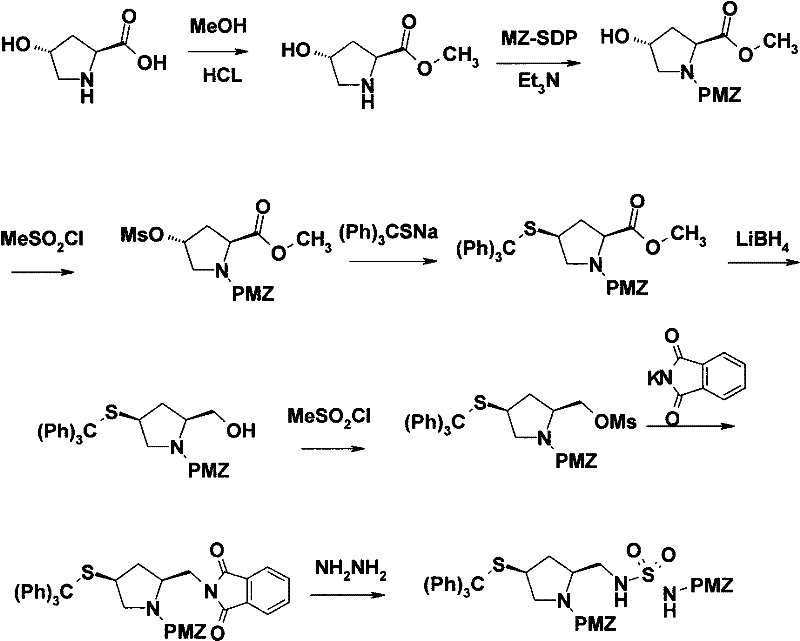
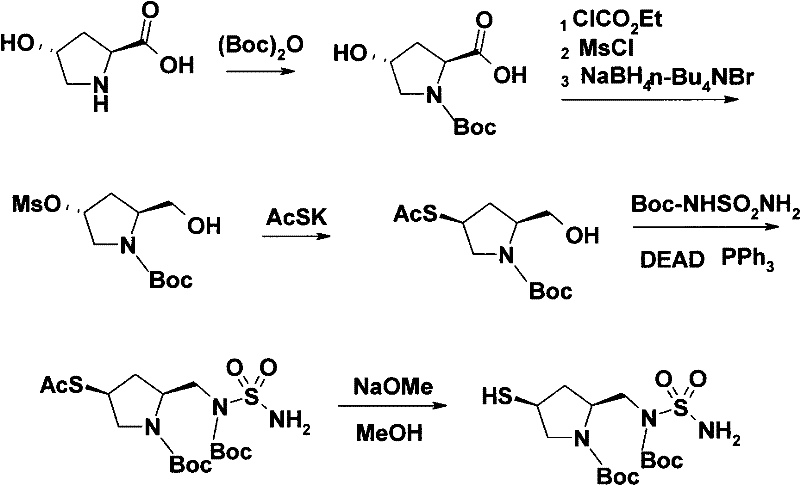
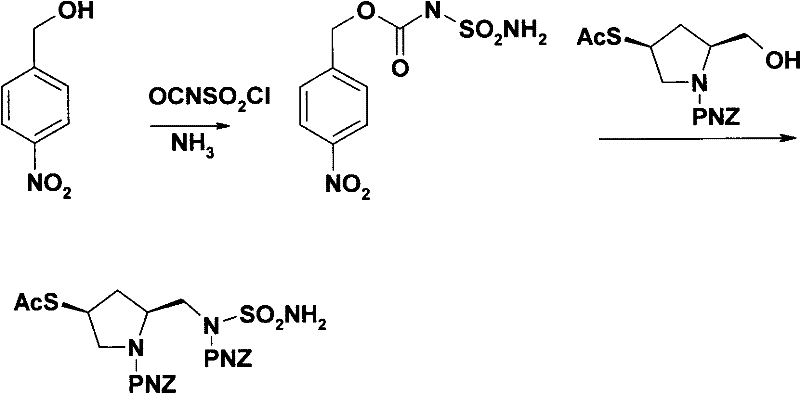
[0036] The present invention will be further described below in conjunction with specific embodiment:
[0037] Amino protection. In a 200L reaction kettle, dissolve 12.4kg of L-hydroxyproline and 13.6kg of sodium hydroxide in 120L of water, cool to 0°C, add dropwise 41kg of 50% p-nitrobenzyl chloroformate in toluene, at 0~5°C Stir for 1 hour, separate the water layer, adjust the pH of the water layer to 2 with concentrated hydrochloric acid, precipitate a solid, centrifuge, and dry to obtain 25.6 kg of white solid.
[0038] Carboxyl protection. In a 200L reaction kettle, dissolve 20kg of (2S,4R)-1-p-nitrobenzyloxycarbonyl-2-carboxy-4-hydroxypyrrolidine in 30L of methanol, add 1.2kg of concentrated sulfuric acid, heat up to 65°C and stir and reflux for 7h. After concentration, 100 L of ethyl acetate and 100 L of saturated sodium chloride solution were added, and the organic layer was separated, dried, and concentrated to obtain an oil, which was directly put into the next rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com