Stable protein kinase activator, and preparation method and purpose thereof
A protein kinase and activator technology, applied in the field of medicine, can solve the problem of no protein kinase activator - cyclic adenosine monophosphate preparation method and use, etc., and achieve the effect of good storage stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Example 1 Preparation of magnesium salt 8 hydrate of cyclic adenosine monophosphate
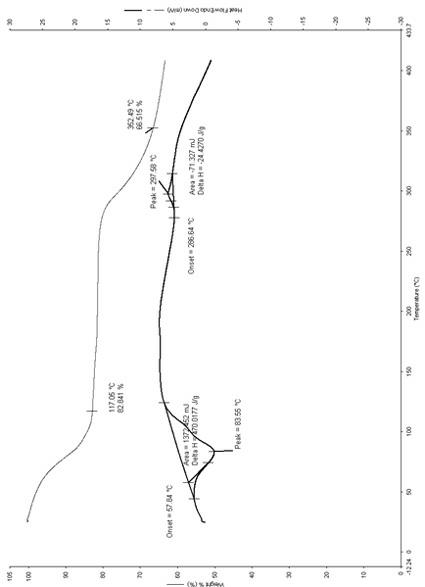
[0078] In a 250ml three-necked flask, add 0.1mol of cyclic adenosine monophosphate, 100ml of water, stir, add 0.05mol of magnesium oxide, stir at 20-60°C for about 50 minutes to dissolve, filter, add 5 times the amount of acetone to make the solid Precipitation, cooling to about -10°C, standing for 24 hours, filtering, drying the solid at about 50°C for 4 hours to obtain 23.6g of off-white solid, melting point: 227°C decomposition (uncorrected), HPLC: cyclic adenosine monophosphate content 39.86%, Moisture is 17.57% as measured by Karl Fischer method, and thermal analysis: the weight loss of the platform is about 17.16% (see attached figure 1 ), which is within the error range with the result of the sample containing 8 crystal waters (theoretical value 17.48%); infrared spectrum: ν KBr max cm -1 3437, 3210, 2913, 2428, 1659, 1606, 1581, 1488, 1425, 1384, 1343, 1290, 1237, 1136, 1...
Embodiment 2
[0079] Example 2 Preparation of Cyclic Adenosine Monophosphate Calcium Salt 3.5 Hydrate
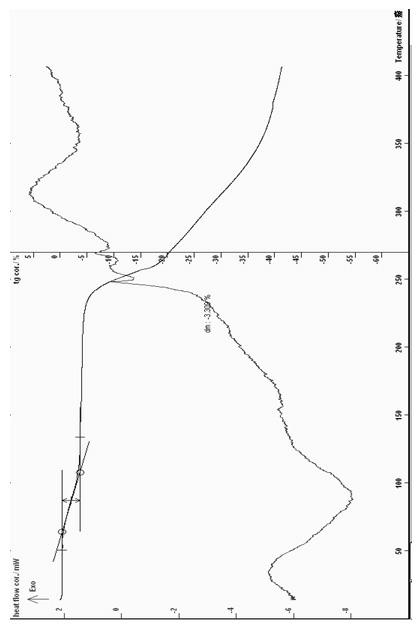
[0080] In a 250ml three-necked flask, add 0.1mol of purified cyclic adenosine monophosphate, 100ml of water, stir, add 0.05mol of calcium hydroxide or calcium oxide, stir at 20-60°C for about 60 minutes to dissolve, filter, Add 5 times the amount of absolute ethanol and isopropanol (1:1), cool to -10 ~ 5 ° C, stand for 24 hours, wait for the solid to precipitate, filter, and dry at about 50 ° C for 4 to 6 hours to obtain an off-white solid 19.2 grams, melting point: decomposition at 223°C (ELECTROTHERMAL MELTING POINT APPARATUS, uncorrected), infrared spectrum: Karl Fischer’s method determines that the water content is 8.52%, thermal analysis: the weight loss on the platform is about 8.4%, which is consistent with the result that the sample contains 3.5 crystal waters ( Theoretical value 8.30%) within the error range; ESI: m / z: 328; IR spectrum: ν KBr max cm -1 3436, 3209, 2913, 2428...
Embodiment 3
[0081] Example 3 Preparation of adenosine cyclophosphate meglumine monohydrate
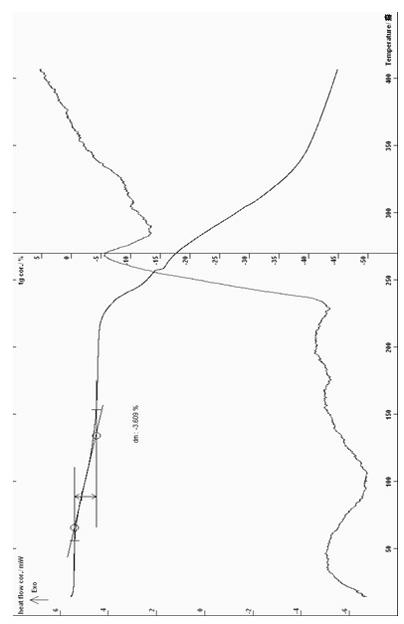
[0082] Put 10g of purified cyclic adenosine monophosphate and equimolar meglumine into the reaction bottle, add 200ml of water, stir, control the temperature between 10-60°C, react for 10-120min, add 1% of the total solution Stir the activated carbon for half an hour and filter it, then filter it with a 0.22 micron microporous membrane, freeze it to -60~-40°C, keep it for about 4 hours, then raise the temperature to about -16°C, vacuum between 5-12Pa, vacuum Dry for about 20 hours, then vacuum-dry for about 4 hours at 20-30°C and vacuum degree 5-12Pa to obtain a white solid, which is easily soluble in water and has a melting point of 67-71.°C (ELECTROTHERMAL MELTING POINT APPARATUS , uncorrected); HPLC: cyclic adenosine monophosphate content is 60.79%, ESI: m / z: 523,328, 19,6, 195; moisture (Karl Fischer method): 3.51%, thermal analysis: platform weight loss is about 3.31%, which The result with ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com