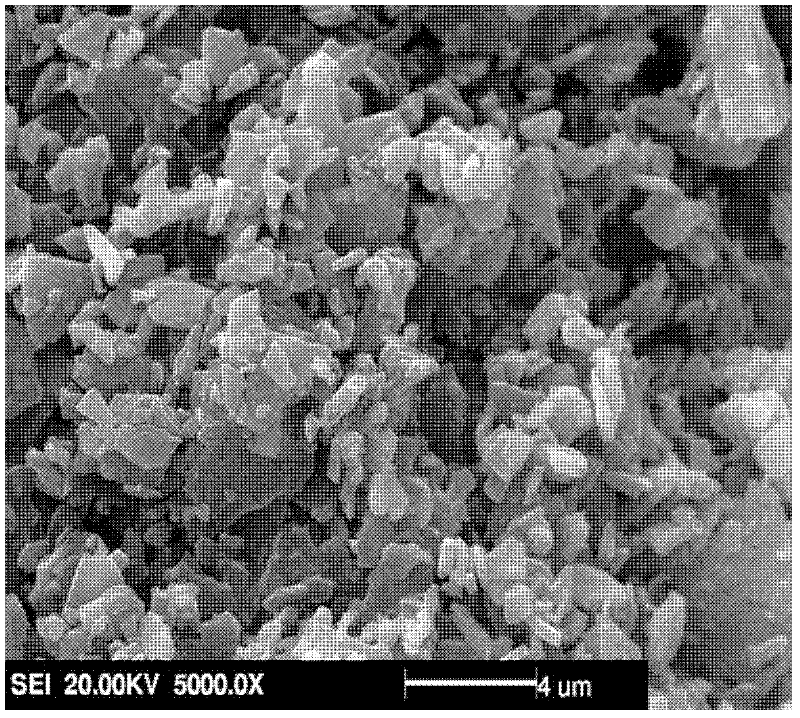
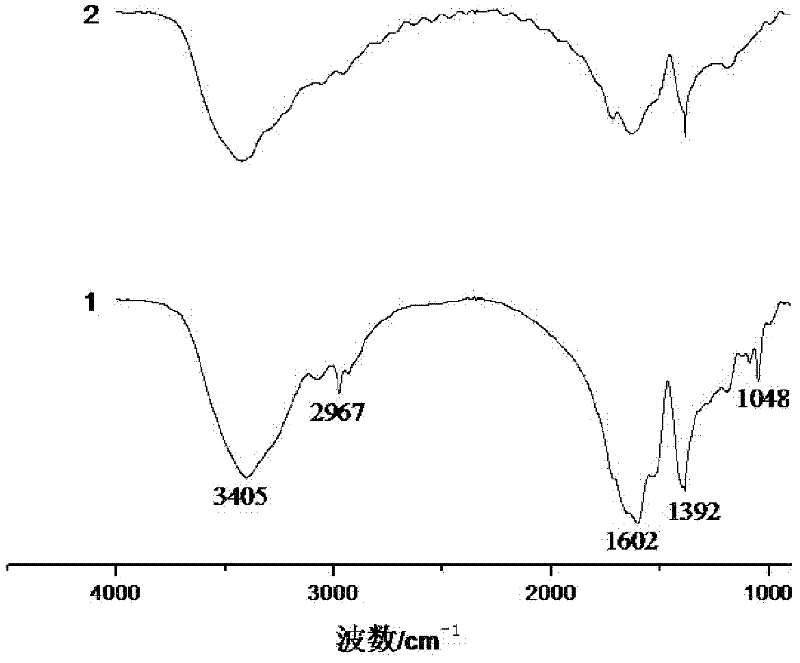
Method for synthesizing polyaspartic acid derivative through multi-component evaporative crystallization copolymerization modification
A technology of polyaspartic acid and copolymerization modification is applied in the field of synthesis of polyaspartic acid derivatives, which can solve the problems of high crystallinity, low scale inhibition rate of polyaspartic acid, and polyaspartic acid particles. Large diameter and other problems, to achieve the effect of reducing crystallinity and excellent scale inhibition performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0010] Specific embodiment 1: The method for synthesizing polyaspartic acid derivatives by multi-component evaporative crystallization copolymerization modification in this embodiment is carried out according to the following steps: 1. Pretreatment: mixing L-aspartic acid and L-serine amino acid Dissolve in distilled water to make a mixed solution, heat and stir the mixed solution at 200°C until the liquid is completely evaporated, and the solid is precipitated, and then the solid is dried in a constant temperature oven at 80°C to constant weight to obtain a sample; 2. Take steps 1. Heating the prepared sample to 180-240°C at a heating rate of 2-4°C / min, and then reacting for 1-6h under the condition of a stirring rate of 50-100r / min to obtain polysuccinimide derivatives; 3. After cooling the polysuccinimide derivative to room temperature, add 0.5mol / L sodium hydroxide solution dropwise until the pH value is 10-12 to obtain a polyaspartic acid sodium salt solution; 4. Add hydr...
specific Embodiment approach 2
[0013] Embodiment 2: The difference between this embodiment and Embodiment 1 is that the mass concentration of L-aspartic acid in the mixed solution in Step 1 is 90%. Others are the same as in the first embodiment.
specific Embodiment approach 3
[0014] Embodiment 3: The difference between this embodiment and Embodiment 1 or 2 is that the mass concentration of L-serine amino acid in the mixed solution in Step 1 is 10%. Others are the same as in the first or second embodiment.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com