Daidzein solid lipid nanoparticles and preparation method thereof
A technology of solid lipid nanometer and daidzein, which can be used in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve problems such as poor solubility of daidzein, and achieve oral bioavailability. The effect of increasing the degree of metabolism, reducing the metabolism, and increasing the adhesion ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
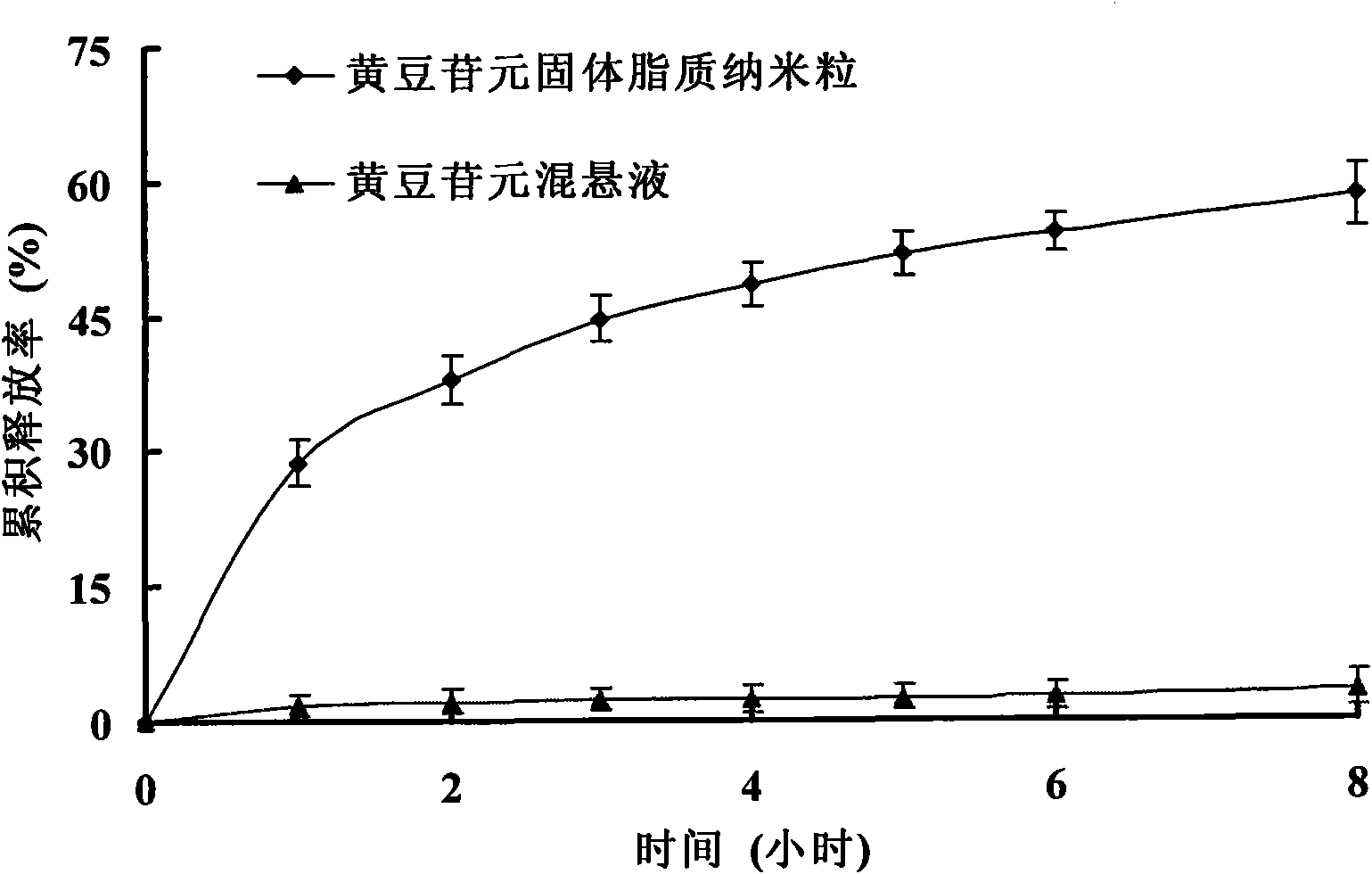
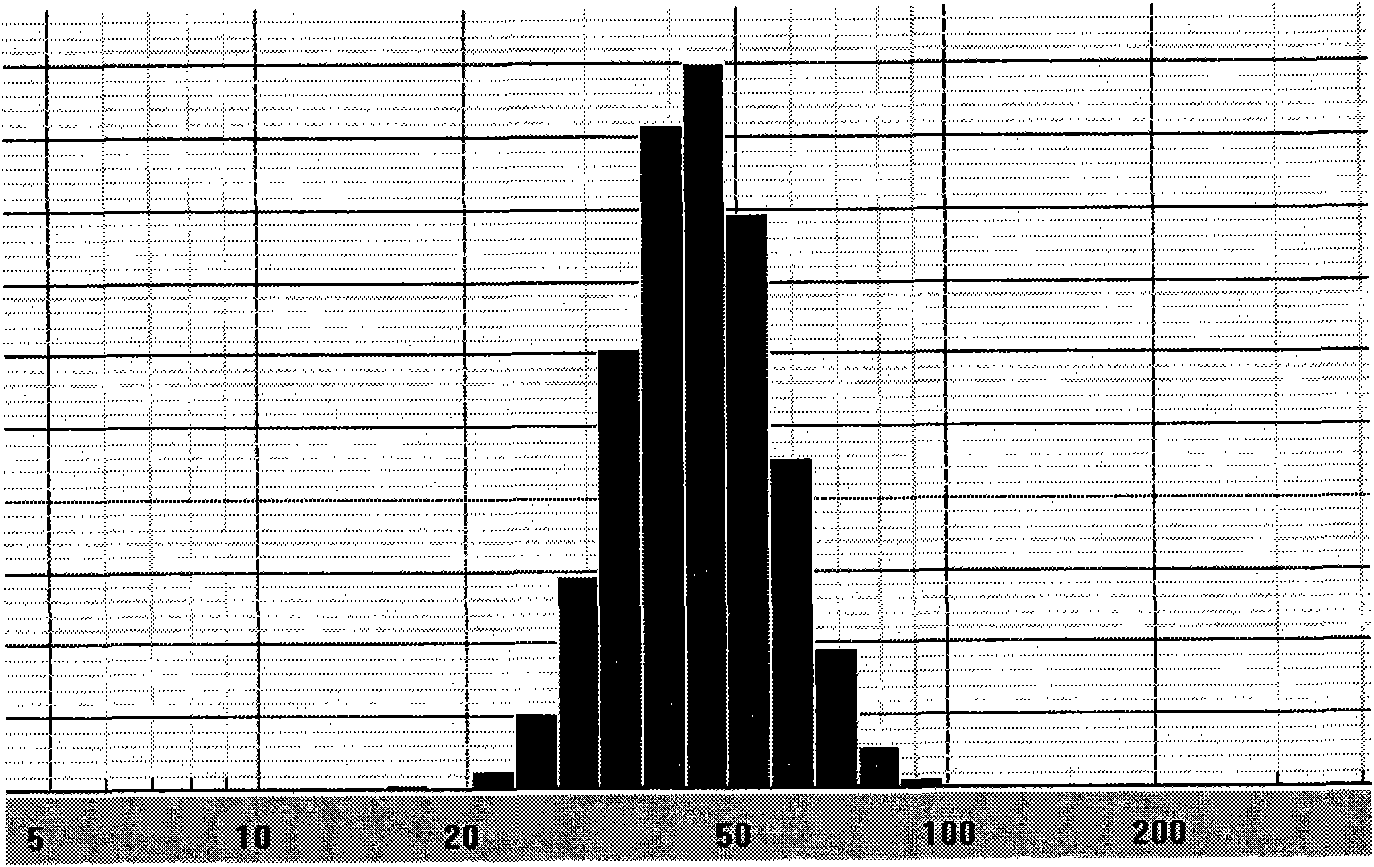
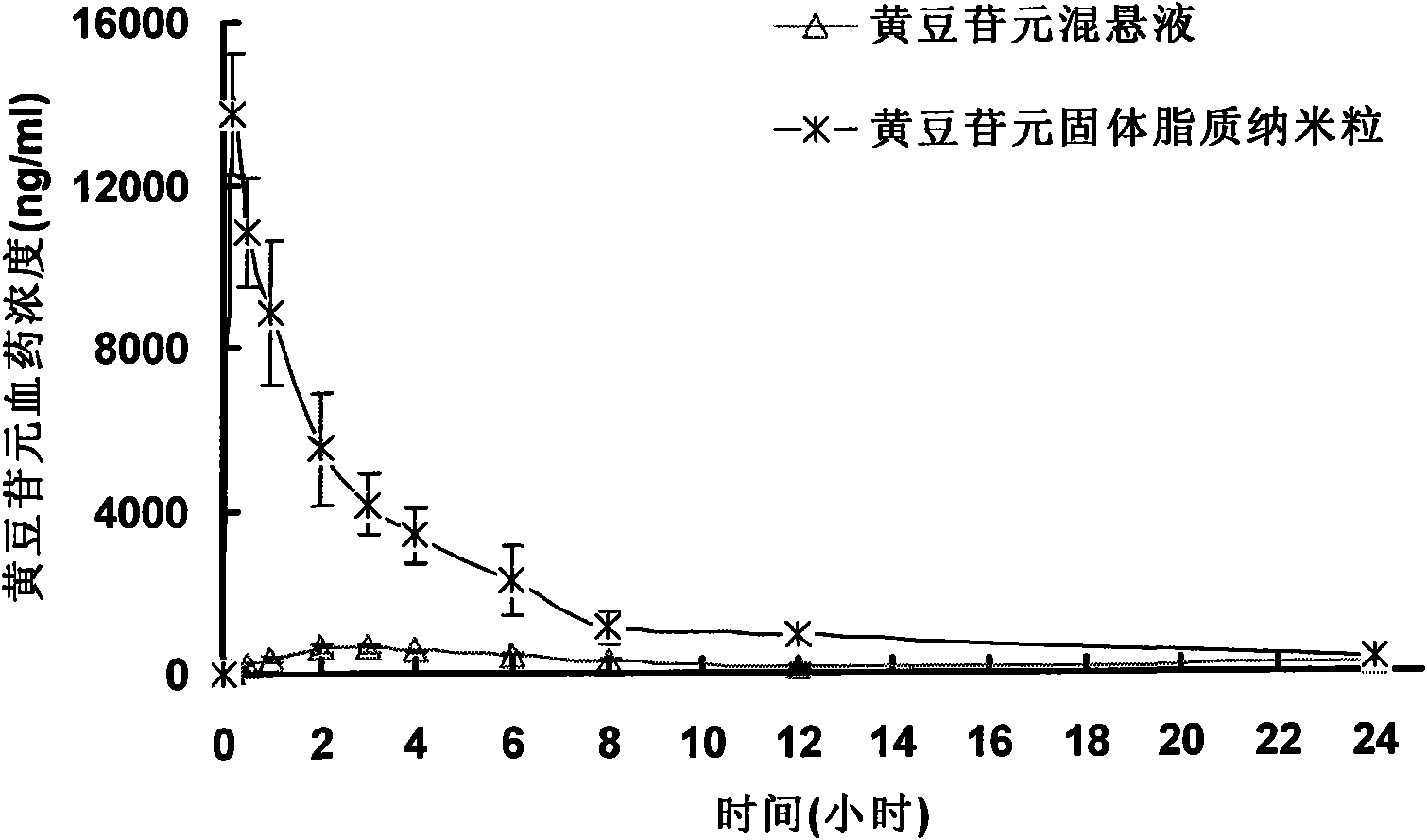
Image
Examples
Embodiment 1
[0069] composition:
[0070] Daidzein 100mg
[0071] Soy Lecithin 1.0g
[0072] Sodium Oleate 1.0g
[0073] Glyceryl Behenate 0.2g
[0074] Preparation method: It is prepared by thin film ultrasonic dispersion method, and the specific preparation operation is as follows:
[0075] Take 100 mg of daidzein and 0.5 g of soybean lecithin, add 100 mL of methanol, reflux under reduced pressure at 50 ° C, while maintaining the temperature and magnetic stirring for 2 hours, then remove the organic solvent by rotary evaporation under reduced pressure to obtain a solid residue, and dry it in vacuum for 12 hours. Obtain the complex of daidzein and phospholipid;
[0076] Add 0.5g of soybean lecithin, 1.0g of sodium oleate, and 0.2g of glyceryl behenate to the complex of daidzein and phospholipid, dissolve them in 20mL of dichloromethane, and then rotary evaporate to form a thin film; 20mL of distilled water preheated to 50°C was shaken horizontally in a 50°C water bath for 5 minutes t...
Embodiment 2
[0079] composition:
[0080] Daidzein 100mg
[0082] Sodium Oleate 1.0g
[0083] Glyceryl monostearate 0.5g
[0084] Preparation method: It is prepared by thin film-high pressure homogeneous method, and the specific preparation operation is as follows:
[0085] Take 100 mg of daidzein and 0.5 g of egg yolk lecithin, add 100 mL of methanol, reflux under reduced pressure at 50 ° C, while maintaining the temperature and magnetic stirring for 4 hours, then remove the organic solvent by rotary evaporation under reduced pressure to obtain a solid residue, and dry it in vacuum for 8 hours. Obtain the complex of daidzein and phospholipid;
[0086] Add 0.5g of egg yolk lecithin, 1.0g of sodium oleate, and 0.5g of glyceryl monostearate to the complex of daidzein and phospholipids, dissolve in 50mL of methanol, and then rotary evaporate to form a film; add 20mL of pre- Heat distilled water at 50°C, shake horizontally in a water bath at 50°C for 5 minutes ...
Embodiment 3
[0089] composition:
[0090] Daidzein 100mg
[0091] Hydrogenated Soy Lecithin 1.0g
[0092] Tween 80 1.0g
[0093] Stearic acid 0.5g
[0094] Preparation method: It is prepared by hot-melt-high-pressure homogeneous method, and the specific preparation operation is as follows:
[0095] Take 100 mg of daidzein and 0.6 g of hydrogenated soybean lecithin, add 100 mL of methanol, reflux under reduced pressure at 50 ° C, and maintain the temperature with magnetic stirring for 6 hours, then remove the organic solvent by rotary evaporation under reduced pressure to obtain a solid residue, and dry it in vacuum for 10 hours , to obtain the complex of daidzein and phospholipid;
[0096] Add 0.4g of hydrogenated soybean lecithin to the complex of daidzein and phospholipid, dissolve it in 5mL of chloroform, then add it into molten stearic acid (0.5g, 80°C), mix well, stir continuously, and evaporate the organic solvent , to obtain a uniformly mixed solid lipid mixture;
[0097] At t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com