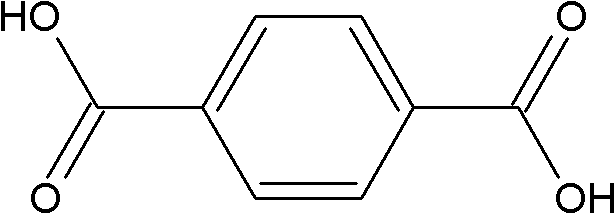
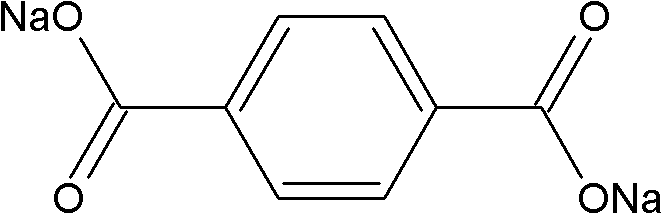
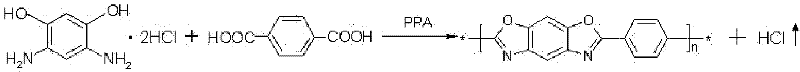Preparation method of poly-p-phenylene benzobisoxazole fiber
A technology of benzobisoxazole and polyparaphenylene, applied in the field of polymer fiber preparation, can solve the problems of increased process difficulty, increased side reactions, and high requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]3117.4 grams of terephthalic acid and 1502.3 grams of sodium hydroxide were dissolved in 15000 grams of water to prepare an aqueous solution of sodium terephthalate with a concentration of 20.1 wt%. Under normal temperature, nitrogen atmosphere and stirring conditions, 4000 grams of solid powdery 4,6-diamino-1,3-resorcinol hydrochloride was added to the above-mentioned sodium terephthalate aqueous solution within 20 minutes, and the addition was completed. After that, the temperature of the system was raised to 80°C within 20 minutes, and a large amount of white precipitate appeared. The white precipitate was 4,6-diaminoresorcinol / terephthalic acid compound salt, and continued at 80°C The reaction was stirred for 30 minutes. Then filter, and weigh the filtered water removed, which is 13324 grams (accounting for 85% of the total water weight of the system); 2352 grams of water remain in the system. Under nitrogen protection, add 16170 grams of polyphosphoric acid and 138...
Embodiment 2
[0041] 3,117.4 grams of terephthalic acid and 1,502.3 grams of sodium hydroxide were dissolved in 21,500 grams of water to prepare an aqueous solution of terephthalic acid sodium salt with a concentration of 15.1 wt%. Under normal temperature, nitrogen atmosphere and stirring conditions, 4000 grams of solid powdery 4,6-diamino-1,3-resorcinol hydrochloride was added to the above-mentioned sodium terephthalate aqueous solution within 20 minutes, and the addition was completed. Afterwards, the temperature of the system rose to 80° C. within 20 minutes, a large amount of white precipitates appeared, and the stirring reaction was continued at 80° C. for 30 minutes. Filter, and weigh the filtrated water removed, which is 17741 grams (accounting for 80% of the total water weight of the system), and 4439 grams of water remain in the system. Under the protection of nitrogen, 21443 grams of polyphosphoric acid and 19299 grams of phosphorus pentoxide were added to the system, reacted at ...
Embodiment 3
[0045] 3117.4 grams of terephthalic acid and 1502.3 grams of sodium hydroxide were dissolved in 11155 grams of water, and the concentration of the prepared aqueous solution of sodium terephthalic acid salt was 25.0 wt%. Under normal temperature, nitrogen atmosphere and stirring conditions, 4000 grams of solid powdery 4,6-diamino-1,3-resorcinol hydrochloride was added to the above-mentioned sodium terephthalate aqueous solution within 20 minutes, and the addition was completed. Afterwards, the temperature of the system rose to 80° C. within 25 minutes, a large amount of white precipitates appeared, and the stirring reaction was continued at 80° C. for 30 minutes. Filter, and weigh the filtrated water removed, which is 10647 grams (accounting for 90% of the total water weight of the system), and 1184 grams of water remain in the system. Under nitrogen protection, 11424 grams of polyphosphoric acid and 9368 grams of phosphorus pentoxide were added to the system, and 1251 grams re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com