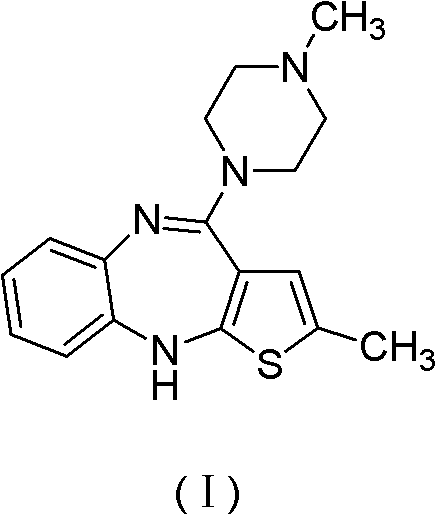
A kind of preparation method and refining method of olanzapine
A refining method, the technology of olanzapine, applied in the direction of organic chemistry, etc., can solve the problems of increasing the difficulty of quality control, limited source of raw materials, increasing production costs, etc., and achieve the effect of high yield, simple process and low pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The preparation of embodiment 1 olanzapine
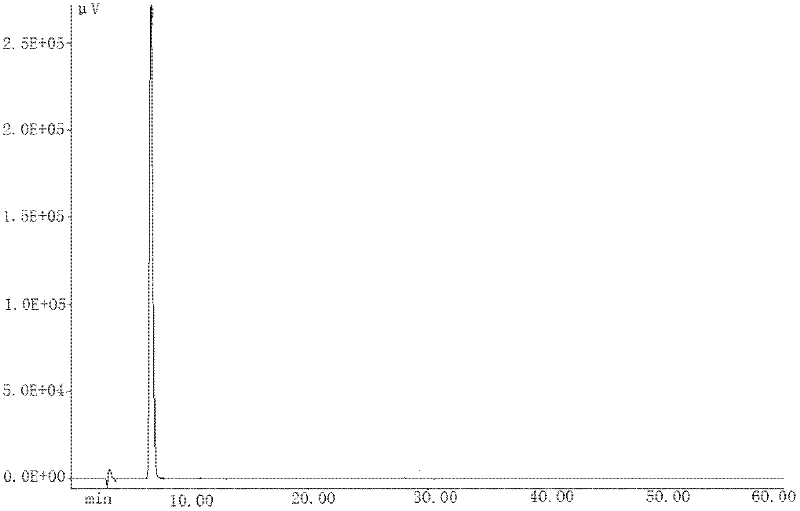
[0031] To a 250ml there-necked flask, add 4-amino-2-methyl-10H-thieno[2,3-b][1,5]-benzodiazepine hydrochloride 20.00g (0.075mol), N- Methylpiperazine 75.30 g (0.75 mol) was stirred with nitrogen and heated to reflux for 2 h. The reaction solution was poured into 200 ml of water with stirring, and a light yellow powder solid was precipitated, which was continued to stir for 1 h, filtered and dried to obtain 23.30 g of olanzapine product with a yield of 99.4% and a purity of 99.0% (HPLC).
Embodiment 2
[0032] The purification of embodiment 2 olanzapine
[0033] The 23.30g olanzapine product obtained in Example 1 was transferred into a 250ml single-neck bottle, 240ml of ethanol was added, stirred, and heated to reflux to completely dissolve the product. 0.20g of activated carbon was added to the system, refluxed for decolorization for 30min, filtered, the filtrate was cooled to room temperature for crystallization, filtered and dried to obtain 16.32g of yellow crystalline powder with a yield of 70.0% and a purity of 99.8% (HPLC). .
Embodiment 3
[0034] The preparation of embodiment 3 olanzapine
[0035] Add 4-amino-2-methyl-10H-thieno[2,3-b][1,5]-benzodiazepine hydrochloride 150.03g (0.56mol), N- Methylpiperazine 339.29 g (3.39 mol) was stirred with nitrogen and heated to reflux for 2 h. The temperature is lowered, and more than 70% excess N-methylpiperazine is recovered by distillation under reduced pressure to obtain the solid crude olanzapine.
[0036] Add 400 ml of ethanol to the three-necked flask, and heat to reflux to completely dissolve the solid. The ethanol solution of olanzapine was then poured into 1000 ml of water under stirring, and a light yellow powder solid was precipitated, which was filtered and dried to obtain 173.87 g of olanzapine product with a yield of 99.4% and a purity of 98.9% (HPLC).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com