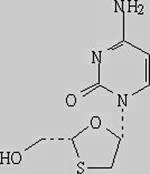
Lamivudine preparation and preparation method thereof
A technology for lamivudine and preparations, which is applied to pharmaceutical formulations, antiviral agents, and pill delivery, and can solve problems such as low initial dissolution rate, poor content uniformity, and uneven particle size distribution of drug-containing particles. Simple and easy-to-operate effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
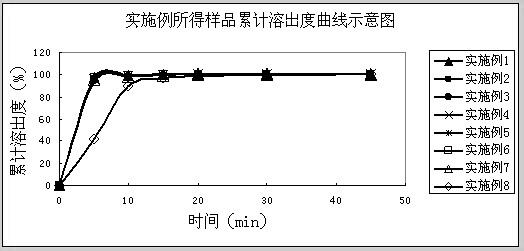
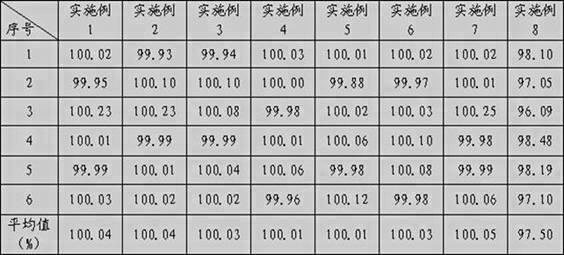
Image
Examples
Embodiment 1
[0026] components weight Lamivudine 100g microcrystalline cellulose 20g lactose 10g Sodium carboxymethyl starch 3g 2.5% ethanol solution of povidone 30ml Micropowder silica gel 1g Magnesium stearate 1g
[0027] Preparation:
[0028] (1) Separately sieve lamivudine, lactose, microcrystalline cellulose, and sodium carboxymethyl starch, and mix well to obtain a mixed powder;
[0029] (2) Add 2.5% povidone ethanol solution into the mixed powder to make soft material, granulate, granulate after drying, add micropowder silica gel and magnesium stearate, and mix well;
[0030] (3) Packed into capsules or compressed tablets;
Embodiment 2
[0032] components weight Lamivudine 100g pregelatinized starch 15g lactose 8g Low-substituted hydroxypropyl cellulose 2g 2.5% ethanol solution of povidone 20ml Micropowder silica gel 1g Magnesium stearate 1g
[0033] Preparation method is the same as embodiment 1
Embodiment 3
[0035] components weight Lamivudine 100g microcrystalline cellulose 30g lactose 15g Croscarmellose Sodium 5g 2.5% ethanol solution of povidone 40ml Micropowder silica gel 2g
[0036] Preparation method is the same as embodiment 1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com