N-(2-acetic acid) salicylhydrazone rare earth complex and its preparation method and use
A salicylhydrazone rare earth and salicylhydrazone technology are applied in the fields of N-(2-acetic acid) salicylhydrazone rare earth complexes and their preparation and use, and achieve the effects of strong coordination ability, good bacteriostatic activity and excellent biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
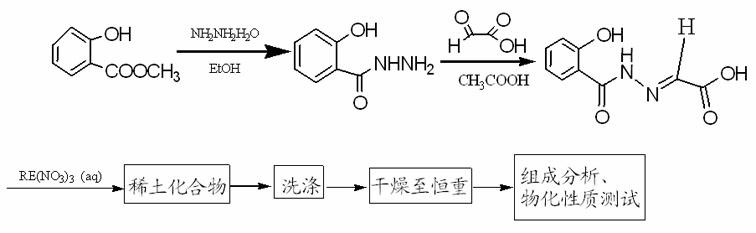
[0025] Example 1 N-(2-acetic acid) salicylhydrazone (C 9 h 8 N 2 o 4 ) Synthesis
[0026] Add 46.8mL of methyl salicylate, 22.9mL of hydrazine hydrate and 23.6mL of absolute ethanol into a 250mL round-bottomed flask, stir and reflux in a water bath for 2 hours, transfer to a beaker while hot, and cool naturally, a large number of white crystals appear, after standing still, suction filter , washed with absolute ethanol. Its melting point was measured to be 145-146°C, consistent with the literature value of 145-146°C, and the yield was 72.3%.
[0027] Add 2mL of 50% glyoxylic acid dropwise to 15mL of glacial acetic acid dissolved with 2.4676g of salicylhydrazide, a pale yellow precipitate appears immediately, react in a water bath at 50°C for 1 hour, cool, and filter to obtain a light yellow powdery solid, namely N - Crude (2-acetic acid) salicylhydrazone. The powder was recrystallized with distilled water to obtain white blocky crystals. Yield: 75%, melting point 2...
Embodiment 2
[0035] Example 2 Nd(C 9 h 7 N 2 o 4 )(C 9 h 6 N 2 o 4 ) 2H 2 Synthesis of O
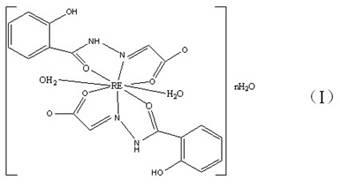
[0036] Weigh 1.5mmol Nd(NO 3 ) 3 4H 2 O and 3.0mmol N-(2-acetic acid) salicylhydrazone (i.e. C 9 h 8 N 2 o 4), were dissolved in 100ml water respectively, and the aqueous solution of N-(2-acetic acid) salicylhydrazone was heated to boiling, and alkali solution was added dropwise to make H 3 L is completely dissolved. Stop heating, and immediately add the aqueous solution of neodymium nitrate, at this time, white turbidity appears, and the pH is 1~2. Use lye to adjust the pH of the solution to 5-6, the solution is yellow and turbid, continue to stir and react at room temperature for 2 hours, and filter with suction to obtain a light yellow powder, wash with hot water and ethanol, dry, and constant weight to obtain the product Nd(C 9 h 7 N 2 o 4 )(C 9 h 6 N 2 o 4 ) 2H 2 O, the yield is 87%.
[0037] Elemental Analysis (%): Calculated Nd 24.26 C 36.36 H 3.05 N 9.42
[003...
Embodiment 3
[0045] Example 3 Y(C 9 h 7 N 2 o 4 )(C 9 h 6 N 2 o 4 ) 4H 2 Synthesis of O:
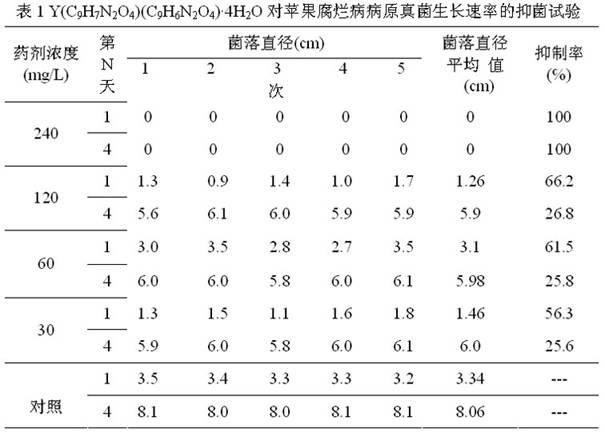
[0046] Weigh 1.5mmol Y(NO 3 ) 3 ·3H 2 O, 3.0mmol N-(2-acetic acid) salicylhydrazone (i.e. C 9 h 8 N 2 o 4 ), were dissolved in 100ml water respectively, the aqueous solution of N-(2-acetic acid) salicylhydrazone was heated to boiling, and alkali solution was added dropwise to make H 3 L is completely dissolved. Stop heating, and immediately add the aqueous solution of yttrium nitrate, at this time, white turbidity appears, and the pH is 1~2. Use lye to adjust the pH of the solution to 5-6. At this time, the solution is yellow and turbid. Continue to stir and react at room temperature for 2 hours, and filter with suction to obtain a light yellow powder. Wash with distilled water and ethanol, dry, and constant weight to obtain Y(C 9 h 7 N 2 o 4 )(C 9 h 6 N 2 o 4 ) 4H 2 O product, the productive rate is 86%.
[0047] Elemental Analysis (%): Calculated Y 15.45 C 37.58 H 3.85 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com