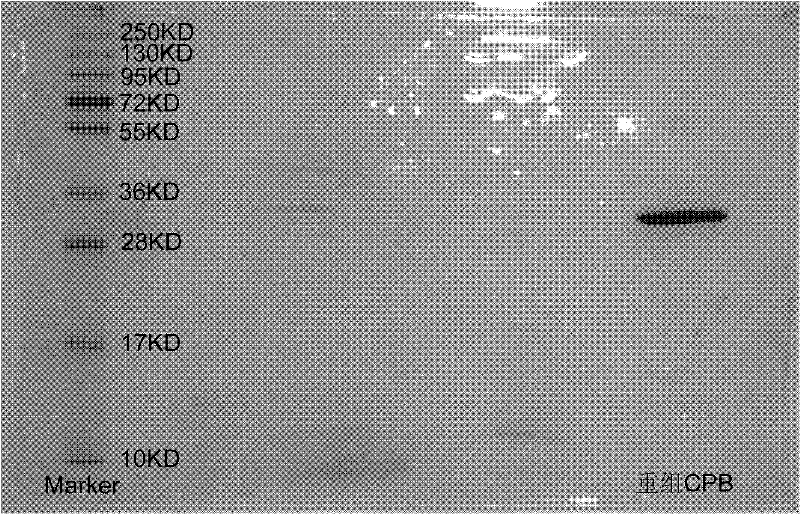
Preparation method of recombinant carboxypeptidase b
A technology of carboxypeptidase and zymogen, which is applied in the field of preparation of recombinant carboxypeptidase B in yeast, can solve the problems of complex process steps and large liquid volume, and achieve the effect of increasing expression and small volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The structure of embodiment 1 recombinant CPB zymogen expression vector
[0041] 1. Provide the nucleotide sequence encoding the CPB zymogen-histidine tag: chemically synthesize the gene sequence encoding the CPB zymogen according to the codon preference of Pichia pastoris, and add 6 groups of codes at the 3' end of the gene sequence Amino acid codon, the final nucleotide sequence is the nucleotide sequence shown in SEQ ID NO:1.
[0042] 2. Using the above-mentioned synthetic nucleotide sequence as a template, and using the designed two primers as upstream and downstream primers, carry out PCR amplification, wherein,
[0043] The primer sequences are:
[0044] Upstream primers:
[0045] GGCGAATTCCATCACCACCATCATCACACTACTGGTCATTCTTACGAGAA
[0046] Downstream primers:
[0047] ATATAGCGGCCGCTTACAATGACCCAAAACGTAGTTA
[0048] The PCR system is:
[0049] Template (5ng / ul) 0.5ul,
[0050] Upstream primer Y-pro-cpb-F (10uM) 0.5uL,
[0051] Downstream primer Y-cpb-3Hi...
Embodiment 2
[0068] The construction of embodiment 2 recombinant CPB zymogen yeast engineering bacteria
[0069] According to the Pichia operation manual of Invitrogen Company, the linearized recombinant CPB zymogen expression vector was electrotransformed into GS115 Pichia competent cells. Then the transformed GS115 Pichia pastoris was spread on MD solid medium and incubated at 29°C for 3-4 days.
[0070] Pick transformants from the above MD solid medium, transfer them to 0.25mg / ml YPD G-418, 0.75mg / ml YPD G-418, 3mg / ml YPD G-418 solid medium, and culture at 29°C. To screen for high-copy transformants. After the high-copy transformant grows up, pick a single colony and inoculate it in 5ml MD liquid medium, and cultivate it at 29°C and 200rpm to produce a large amount of recombinant CPB zymogen expression engineering bacteria GS115-proCPB-3his.
Embodiment 3
[0071] Induced expression of embodiment 3 recombinant CPB zymogen expression engineering bacteria
[0072] Inoculate the above-mentioned engineered bacteria into 10ml of YPD liquid medium according to 2% inoculum amount, cultivate at 28°C, 200rpm. Cultivate until OD 600nm At about 8 o'clock, centrifuge, discard the supernatant, resuspend the bacteria with 1L BMMY liquid medium, put it in a 2L shake flask, fill one bottle for every 500ml bacterial solution, seal the bottle mouth with gauze, 28°C, 200rpm, add methanol Culture to 0.7% (V / V), and replenish methanol to 0.7% (V / V) every 24 hours until the induction is completed on the sixth day. The recombinant CPB zymogen is secreted and expressed into liquid medium.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com