Anti-herpes simplex virus polypeptide and its application
A herpes simplex virus and herpes simplex technology, applied in antiviral agents, applications, peptides, etc., can solve problems such as virus resistance and poor curative effect, and achieve non-toxic and side effects, significant therapeutic effect, and obvious results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: Preparation of anti-herpes simplex virus polypeptide gene
[0031] Total RNA was extracted from the scorpion tail glands of 40 Tibetan scorpions (Scorpops tibetanus) with Trizol reagent (Invitrogen), and the extraction method was referred to the instructions of the Trizol kit. The Poly A Tract mRNA isolation system (Promega, USA) was used to purify the mRNA, and the Superscript Plasmid System cDNA library construction kit (Gibco / BRL) kit was used to construct the cDNA library of Tibetan lute scorpion venom tissue. The cDNA was cloned into pSPORT1 vector and transformed into E. coli DH5α. Randomly select 2000 clones from the constructed Tibetan piper venom cDNA library, and perform sequence analysis on the sequencing results, using CLUSTAL X 1.8 (Thompson et al., 1997) and PC / GENE (Intelligenetics Inc., Switzerland) software for Homology comparison and signal peptide cleavage site prediction obtained a cDNA gene encoding anti-herpes simplex virus active po...
Embodiment 2
[0032] Example 2: Structural analysis of AHSVP1 polypeptide and its homologous amphipathic polypeptide
[0033] According to the mature peptide sequence FIRKIARLLKRIF of AHSVP1 shown in SEQ ID NO: 1, that is, Phe Ile Arg Lys Ile Ala Arg Leu Leu Lys Arg Ile Phe, the online NPSserver [DSC method (Discrimination of protein Secondary structure Class)] was used to perform binary analysis on it. Secondary structure prediction, and use the software AHTHEPROT 2000 to draw its secondary structure image, see the results figure 2 . The secondary structure diagram shows that AHSVP1 contains 100% α-Helix structure, has a typical amphiphilic α-Helix structure, and contains a large number of basic residues (Arg and Lys) with net positive charges. Then, a large number of point mutations and deletions were performed on the AHSVP1 polypeptide sequence according to the AHSVP1 helical diagram, and the sequences are shown in Table 1.
[0034] Table 1: Anti-herpes simplex virus polypeptide AHSVP...
Embodiment 3
[0037] Example 3 Chemical Synthesis of AHSVP1 Polypeptide and Amphipathic Polypeptide with Homologous Structure
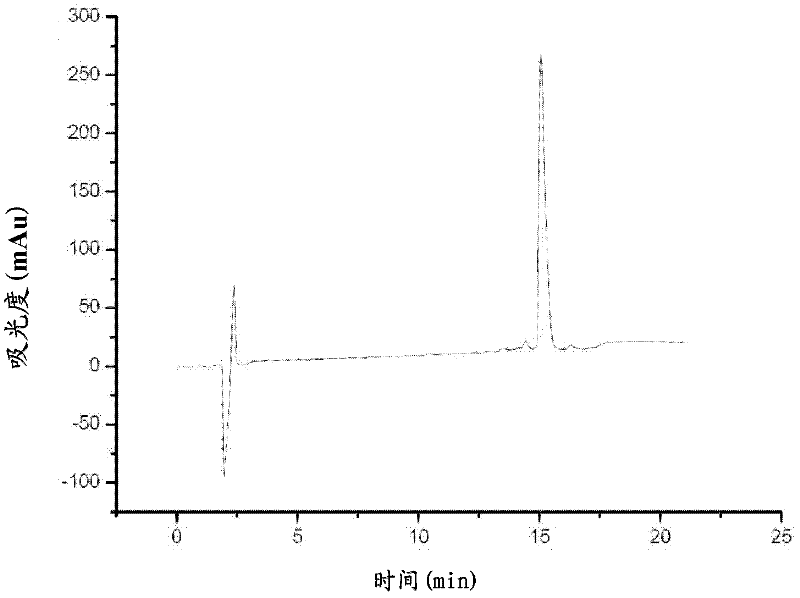
[0038] Artificially synthesized according to the amino acid sequence of AHSVP1 (Phe Ile Arg Lys Ile Ala Arg Leu Leu Lys Arg Ile Phe) and its homologous amphipathic polypeptide. The high-purity AHSVP1 polypeptide and its homologous amphipathic polypeptide were obtained by solid-phase chemical synthesis. The purity test of the prepared AHSVP1 polypeptide was carried out, and the results are shown in image 3 and Figure 4 . The detection results of AHSVP1 homologous structure polypeptides are not shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com