Method for preparing blood coagulation factor ⅷ, fibrinogen and fibronectin by cryoprecipitation
A technology of fibronectin and fibrinogen, which is applied to the preparation methods of fibrinogen and peptides, coagulation/fibrinolytic factors, etc., can solve the problems of insufficient utilization, achieve small batch differences, improve utilization, The effect of high yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
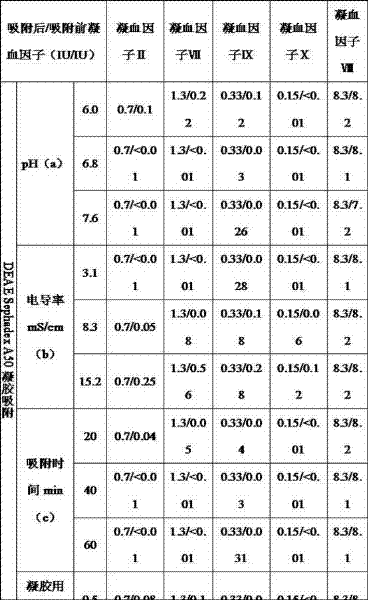
[0041] Step 1: Take 10kg of cryoprecipitate, add 30kg of water for injection at 30°C, stir and dissolve for 1 hour. Add 0.1mol / L acetic acid by spraying at a speed of 120ml / min, adjust the pH value to 6.9, adjust the conductivity to less than 4 mS / cm, and use about 1.4L to remove acetic acid. Add the well-balanced DEAE Sephadex A50 gel, stir and adsorb for 40 minutes, use a continuous flow centrifuge (tube centrifuge) to remove the gel and a small amount of undissolved precipitate, and collect the supernatant.
[0042] The following preparations should be made before the first step: Weigh 20g of DEAE Sephadex A50 gel (dried gel). Place the dry glue in a beaker, add 1L of 80mmol / L sodium chloride solution to swell for 6 hours. Prepare balance solution: 20mmol / L sodium citrate, 60mmol / L sodium chloride, pH 6.9. Discard the upper layer of swelling liquid in the beaker, keep the lower layer of gel, then add 1L of balance solution, stir evenly, and balance for 10-20 minutes, remo...
example 2
[0054] Step 1: Take 10kg of cryoprecipitate, add 50kg of water for injection at 35°C, stir and dissolve for 1 hour; add acetic acid to adjust the pH value to 6.7 by spraying at a speed of 100 ml / min. Add well-balanced DEAE Sephadex A50 gel, stir and absorb for 50 minutes;
[0055] The following preparations should be made before the first step: Weigh 15g of DEAE Sephadex A50 gel (dried gel). Prepare balance solution: 10mmol / L sodium citrate, 80mmol / L sodium chloride, pH 6.7;
[0056] All the other operating steps are the same as the first step of embodiment 1.
[0057] Step 2: Add 0.1 mol / L acetic acid by spraying continuously at a speed of 100 ml / min, and adjust the pH to 6.5. While adding acetic acid, cool down with an ice-water bath, and adjust the temperature to 12°C.
[0058] All the other operating steps are the same as the second step of Example 1.
[0059] The third step: collect 55 kg of the supernatant obtained in the second step, and the operation steps are the ...
Embodiment 3
[0076] Example 3: Research and testing of different adsorption methods and adsorption conditions
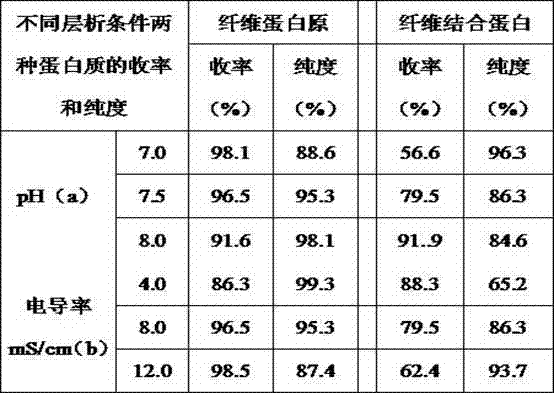
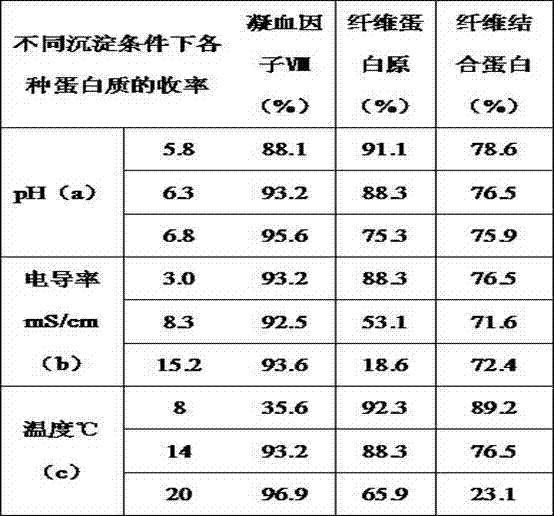
[0077] Many purification processes for coagulation factor VIII and fibrinogen include the step of aluminum hydroxide gel adsorption. Aluminum hydroxide gel can adsorb a variety of coagulation factors, but it is a non-specific adsorbent with limited adsorption capacity. Moreover, the preparation and quality control of aluminum hydroxide gel are more complicated and difficult to control. One of the main reasons why the existing technology cannot make good use of various active ingredients in the cryoprecipitate is that when the coagulation factors II, VII, IX, and X are not completely removed, due to the complicated extraction process of various proteins, The process and time-consuming are relatively long, and a fraction separated from the cryoprecipitate (such as a fraction rich in fibrinogen and fibronectin) will activate coagulation factors and coagulate when used to purify o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com