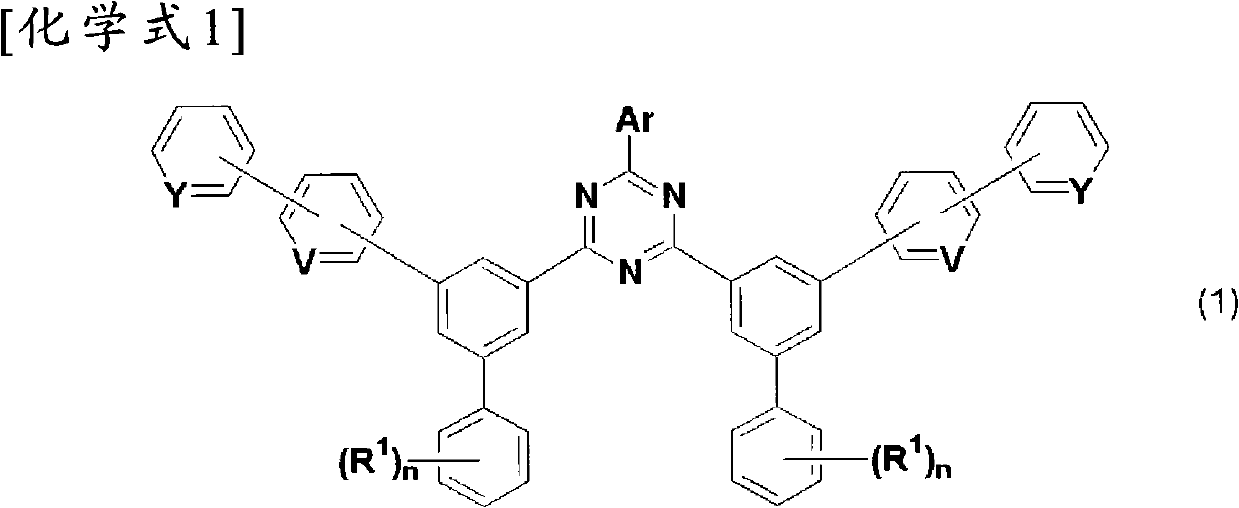
1,3,5-triazine derivatives, preparation method thereof, and organic electroluminescent device using them as constituents
A technology of triazine derivatives and triazine rings, which is applied in the field of organic electroluminescent devices, and can solve problems such as not specifically shown and positions not limited
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
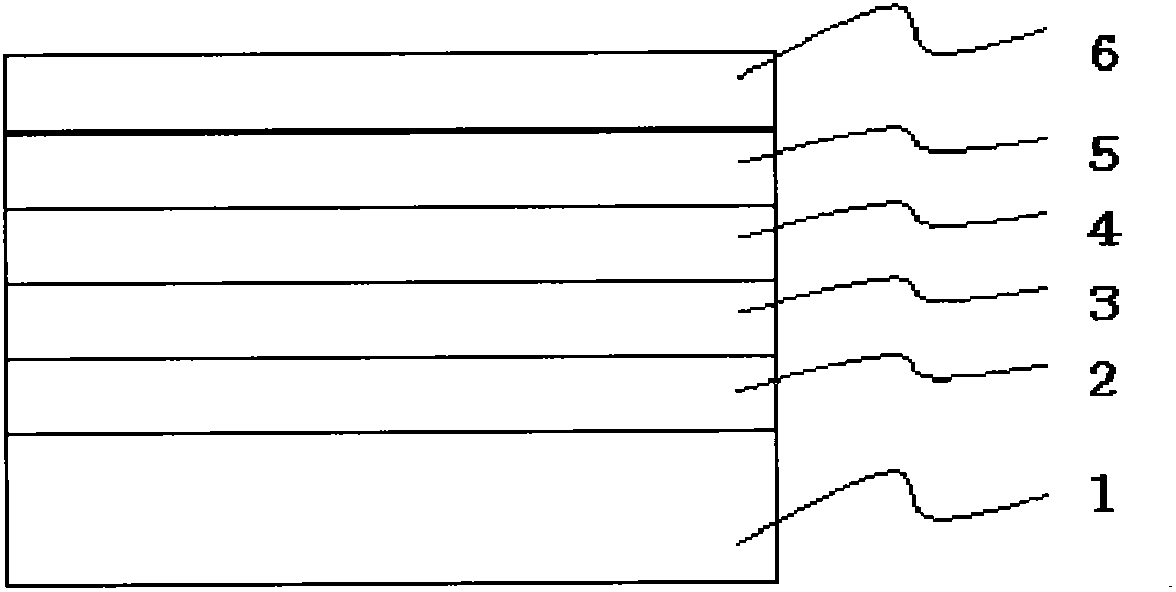
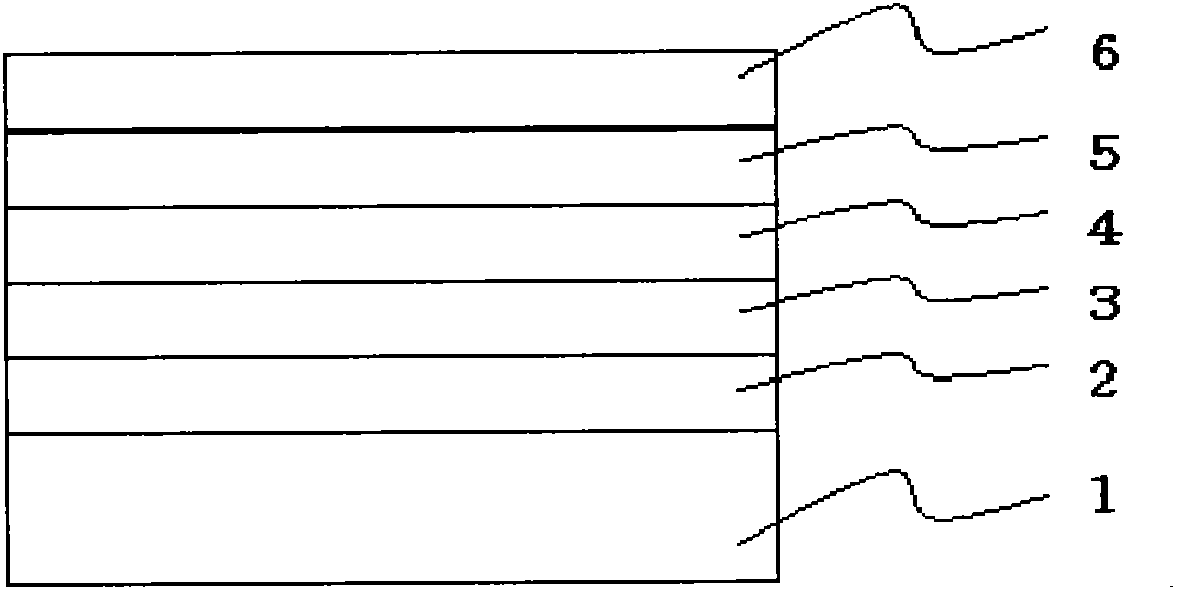
Image
Examples
Embodiment
[0109] The following examples and reference examples are given to illustrate the present invention in more detail, but the present invention is not limited by these examples.
[0110] Reference example-1
[0111]
[0112] Under argon, benzoyl chloride (4.93 g) and 5-chlorobiphenyl-3-carbonitrile (15.0 g) were added to a 300 mL three-necked reaction vessel equipped with a reflux tube, and chlorobenzene (100 mL) was added. The resulting solution was cooled to 0°C, and antimony pentachloride (10.5 g) was added dropwise. The mixture was stirred at room temperature for 20 minutes, and further refluxed at 100° C. for 2 hours. The resulting orange suspension was cooled to -20°C and 28% aqueous ammonia solution (50 mL) was added. After stirring the milky white suspension at room temperature overnight, it was slowly heated to 140° C. using an oil bath, and the organic solvent (65 mL) and water (33 mL) were distilled off. Chlorobenzene (100 mL) was added, heated and filtered at 13...
reference example -2
[0116]
[0117] Under argon, benzoyl chloride (1.23g) and 5-chloro-4'-methylbiphenyl-3-carbonitrile (4.00g) were added to a 200mL three-port reaction vessel equipped with a reflux tube, and chlorobenzene ( 40mL). The resulting solution was cooled to 0°C, and antimony pentachloride (2.63 g) was added dropwise. The mixture was stirred at room temperature for 20 minutes, and further refluxed at 100° C. for 2.5 hours. The resulting red solution was cooled to -20°C, and 28% aqueous ammonia solution (15 mL) was added. After stirring the milky white suspension at room temperature overnight, it was slowly heated to 140° C. using an oil bath, and the organic solvent (25 mL) and water (5 mL) were distilled off. Chlorobenzene (50 mL×2) was added, heated and filtered at 130°C. After cooling the filtrate naturally, methanol (100 mL) was added. The precipitated solid was collected by filtration, washed with methanol (30mL×2), and then dried to obtain 2,4-bis(3-chloro-4'-methylbipheny...
Embodiment -1
[0149]
[0150] Under argon flow, 4-(2-pyridyl)phenylboronic acid (2.25g), 2,4-bis(5-chlorobiphenyl-3-yl)-6-phenyl-1,3,5- Triazine (2.00g), cesium carbonate (3.68g), palladium acetate (33.9mg), 2-dicyclohexylphosphine-2',4',6'-triisopropylbiphenyl (143mg) in tetrahydrofuran (150mL ), and refluxed for 19 hours. After cooling the reaction mixture naturally, low-boiling point components were distilled off under reduced pressure, and methanol was added. The precipitated solid was collected by filtration and purified by silica gel column chromatography (developing solvent methanol:chloroform=1:100~1:75) to obtain the target product 6-phenyl-2,4-bis[4-(2-pyridyl) -1,1';3',1"-terphenyl-5'-yl]-1,3,5-triazine as white powder (yield 2.47 g, yield 85%).
[0151] 1 H-NMR (CDCl 3 ): δ7.26-7.30(m, 2H), 7.45(brt, J=7.3Hz, 2H), 7.53-7.64(m, 7H), 7.79-7.85(m, 8H), 7.93(d, J=8.4 Hz, 4H), 8.12(t, J=1.7Hz, 2H), 8.19(d, J=8.4Hz, 4H), 8.75(brd, J=5.0Hz, 2H), 8.84(brdd, J=7.5, 1.7 Hz, 2H), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com