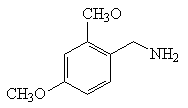
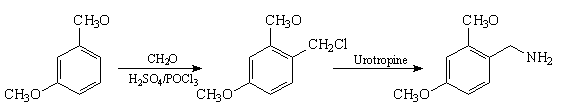
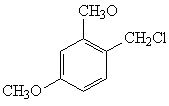
Synthesis method of 2, 4-dimethoxybenzylamine
A technology of dimethoxybenzylamine and dimethoxybenzyl chloride is applied in the field of synthesis of 2,4-dimethoxybenzylamine, which can solve the problems of low position selectivity, difficult control and the like, and achieves operation Safe and simple, low production cost, less three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A kind of synthetic method of 2,4-dimethoxybenzylamine, its synthetic process comprises the following steps:
[0036] (1) Synthesis of 2,4-dimethoxybenzyl chloride
[0037] Add m-xylylene dimethyl ether (70g, 0.5mol), trioctylmethyl ammonium chloride (28g, 0.07mol), paraformaldehyde (20.0g, 0.67mol) and phosphorus oxychloride in 200ml of concentrated sulfuric acid (76%) (191g, 1.25mol), the mixture was stirred at 80°C for 4h, the organic phase was separated after the reaction, the aqueous phase was extracted with hexane (3x200ml), the residue was distilled after the solvent was recovered, and 79.1g of the product (GC>97%) was obtained. Rate 85%; b.p. 81-82℃ / 133.3Pa;
[0038] (2), the synthesis of 2,4-dimethoxybenzylamine
[0039] Hexatropine (42g, 0.3mol), sodium iodide (45g, 0.3mol) and 2,4-dimethoxybenzyl chloride (55.8g, 0.3mol) were added to 1L of ethanol, and the mixture was heated to 40°C at room temperature Stir the reaction for 6 hours, filter the reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com