Composite material used for interbody fusion cage and its preparation method
An intervertebral cage and composite material technology, which is applied in the field of composite materials and preparation of absorbable intervertebral cages, can solve the problems of lack of osteogenic activity, insufficient material crystallinity, and poor strength of the cage, and achieve good bone quality. Induced activity, simple preparation method, and good interfacial bonding effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
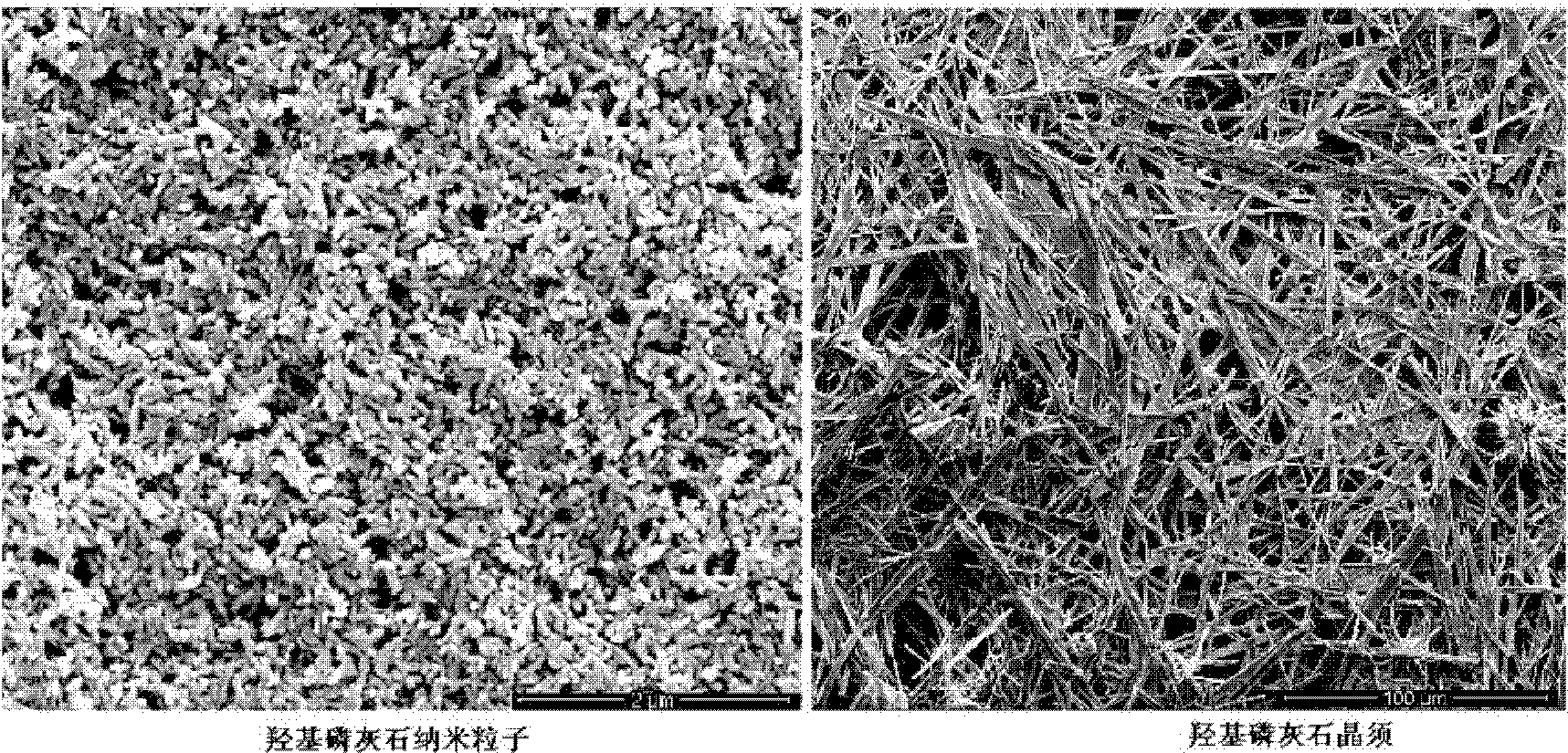
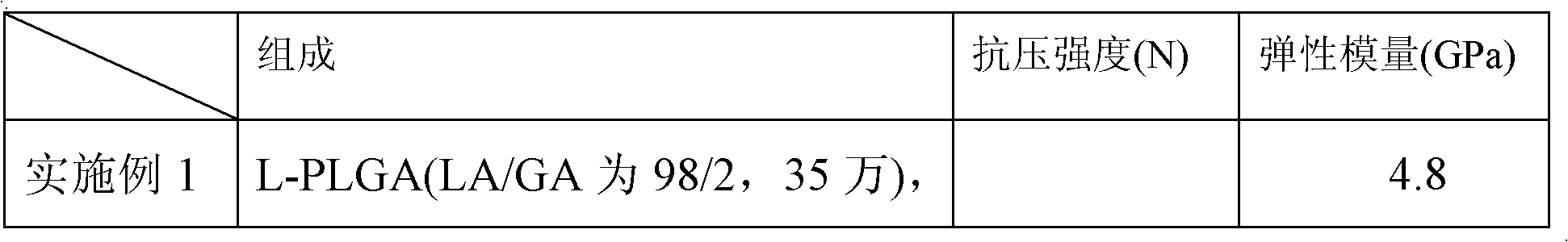
[0024] L-PLGA (the molar ratio of LA / GA is 98 / 2) polymer molecular weight is 350,000, accounts for 95% by weight of finished product, is dissolved in methylene chloride, and concentration is 5g / 100ml; Mixed solvent with dichloromethane (volume ratio: 1:1) was ultrasonically dispersed to form a suspension, and then filtered with 0.22um filter paper. The HA content is measured at 5% of the weight of the finished product, and the L-PLGA solution and the HA solution are mixed under ultrasonic stirring, and the above-mentioned compound solution is continued to be ultrasonically stirred for 2 hours, then precipitated with excess absolute ethanol, left to stand and filtered, and washed with absolute ethanol Washed 3 times and dried in vacuum to obtain L-PLGA / HA composite material.
[0025] The intervertebral fusion cage was made by molding with an injection machine at an injection temperature of 190°C. The intervertebral fusion cage is a hollow cylinder with a quadrangular or hexago...
Embodiment 2
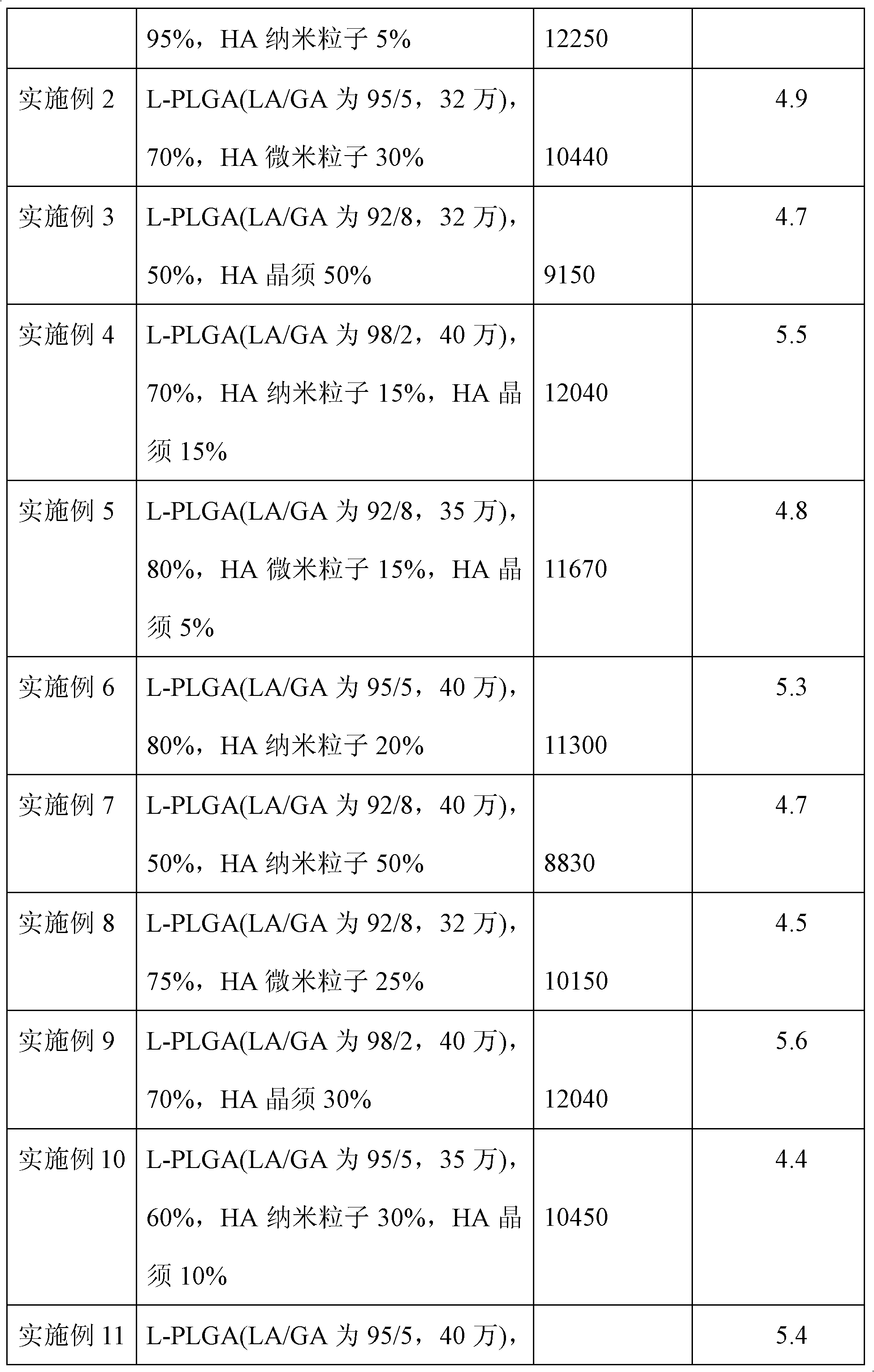
[0027] L-PLGA (the molar ratio of LA / GA is 95 / 5) polymer molecular weight is 320,000, accounts for 70% by weight of finished product, is dissolved in methylene chloride, and concentration is 8g / 100ml; The mixed solvent of dichloromethane (volume ratio is 2: 1) is ultrasonically dispersed into a suspension, filtered with 5um filter paper, and the HA content is 30% based on the weight of the finished product, and added to the dichloromethane solution of L-PLGA under ultrasonic stirring , continue to ultrasonically stir the above composite solution for 3 hours, precipitate with excess absolute ethanol in the above composite solution, stand and filter, wash with absolute ethanol 3 times, and vacuum dry to obtain the L-PLGA / HA composite material.
[0028] Molding by injection machine, injection temperature is 200 ℃, to make intervertebral fusion device. After testing, the finished product has a compression resistance of 11440N and an elastic modulus of 4.9GPa, see Table 1.
Embodiment 3
[0030]L-PLGA (the molar ratio of LA / GA is 92 / 8) polymer molecular weight is 320,000, and HA content accounts for 50% by weight of finished product, is dissolved in methylene chloride, and concentration is 10g / 100ml; The HA whisker After ultrasonically dispersing into a suspension with a mixed solvent of ethanol and dichloromethane (volume ratio is 5:3), filter with 20um filter paper, account for 50% by weight of the finished product, add the dichloromethane solution of L-PLGA under ultrasonic stirring In the process, the above composite solution was continued to be ultrasonically stirred for 4 hours, precipitated with excess petroleum ether in the above composite solution, left to filter, washed 3 times with absolute ethanol, and vacuum-dried to obtain the L-PLGA / HA composite material.
[0031] The intervertebral fusion cage was made by molding with an injection machine at an injection temperature of 210°C. After testing, the finished product has a compression resistance of 10...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com