Semi-synthetic aminoglycoside antibiotic, preparation method and medicine composition thereof
An aminoglycoside and semi-synthetic technology, which is applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems of high production costs, and achieve the effects of high application value, high yield and pure products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (Embodiment 1, 1-N-acetyl minonomycin and preparation method thereof)
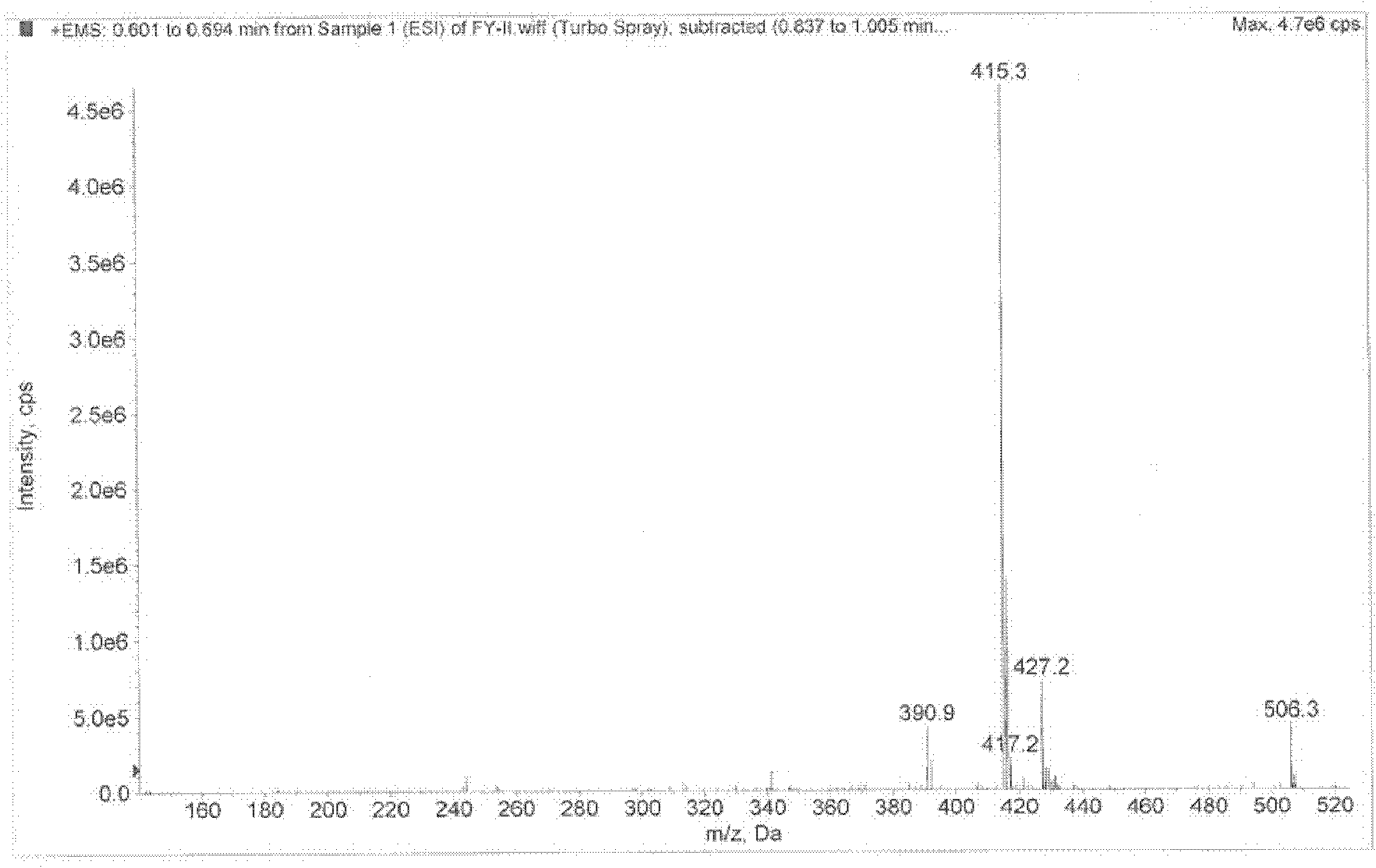
[0039] The semi-synthetic aminoglycoside antibiotic of the present invention is 1-N-acetyl agnonomycin, and molecular formula is C 22 h 44 N 5 o 8 , the molecular weight is 506, and its conformational formula is as follows:
[0040]
[0041] 1-N-Acetyl Daunomycin is produced by semi-synthesis with Daunomycin base (Base) as the mother nucleus.
[0042] The reaction equation for the semi-synthetic preparation of 1-N-acetyldaunomycin with the agnonomycin base as the mother nucleus is as follows:
[0043]
[0044]
[0045] Note: E-MCR refers to 1-N-acetyl minonomycin, and the code name in the efficacy test example is: FY-2.
[0046] The starting material used in the preparation process is micronomycin sulfate, and the CAS number is 66803-19-8. The conformational formula of micronomycin sulfate is as follows:
[0047]
[0048] Described micronomycin sulfate meets the quality standard sti...
Embodiment 2
[0085] (embodiment 2, vitriol 1-N-acetyl agnonomycin and preparation method thereof)
[0086] 1-N-acetyl minonomycin sulfate is a 1-N-acetyl minonomycin molecule with 2.5 H 2 SO 4 Molecules combined, the molecular formula is C 22 h 44 N 5 o 8 ·5 / 2H 2 SO 4 , the molecular weight is 750, and its conformational formula is as follows:
[0087]
[0088] The preparation method of vitriol 1-N-acetyl minomycin is as follows:
[0089] Add the sulfuric acid of 3mol / L in the 50mL of the 1-N-acetyl group phenomycin concentrate obtained in embodiment 1, adjust concentrated solution pH to be 6.0; Add gac 5g to remove heat source again in above-mentioned mixed solution, filter and remove gac to obtain sulfuric acid Concentrate of 1-N-acetyl-minoromycin; then freeze-dry or spray-dry the above-mentioned concentrate to obtain 1-N-acetyl-minoromycin sulfate.
[0090] The prepared 1-N-acetyl minomycin sulfate is a loose solid of white or off-white powder, odorless and hygroscopic; It...
Embodiment 3
[0091] (Embodiment 3, 1-N-acetyl diaunomycin hydrochloride and preparation method thereof)
[0092] The molecular formula of 1-N-acetyl minonomycin hydrochloride is C 22 h 44 N 5 o 8 2HCl, the molecular weight is 578, its conformational formula is as follows:
[0093]
[0094] The rest of the preparation method of 1-N-acetyl minonomycin hydrochloride is the same as Example 2, except that:
[0095] The acid added to 50 mL of the concentrated solution of 1-N-acetyl diaunomycin obtained in Example 1 was 6N hydrochloric acid, and the pH of the concentrated solution was adjusted to be 6.0.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com