Anti-inflammatory inhibitor screening model taking MyD88TIR (myeloid differentiation primary response protein 88 Toll/interleukin-1 receptor) dimerization as target point and application thereof
A technology for inhibitor screening and dimerization, applied in recombinant DNA technology, microbial determination/testing, biochemical equipment and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: GFP-MyD88 TIR and RFP-MyD88 TIR transfected mammalian cells can undergo fluorescence resonance energy transfer
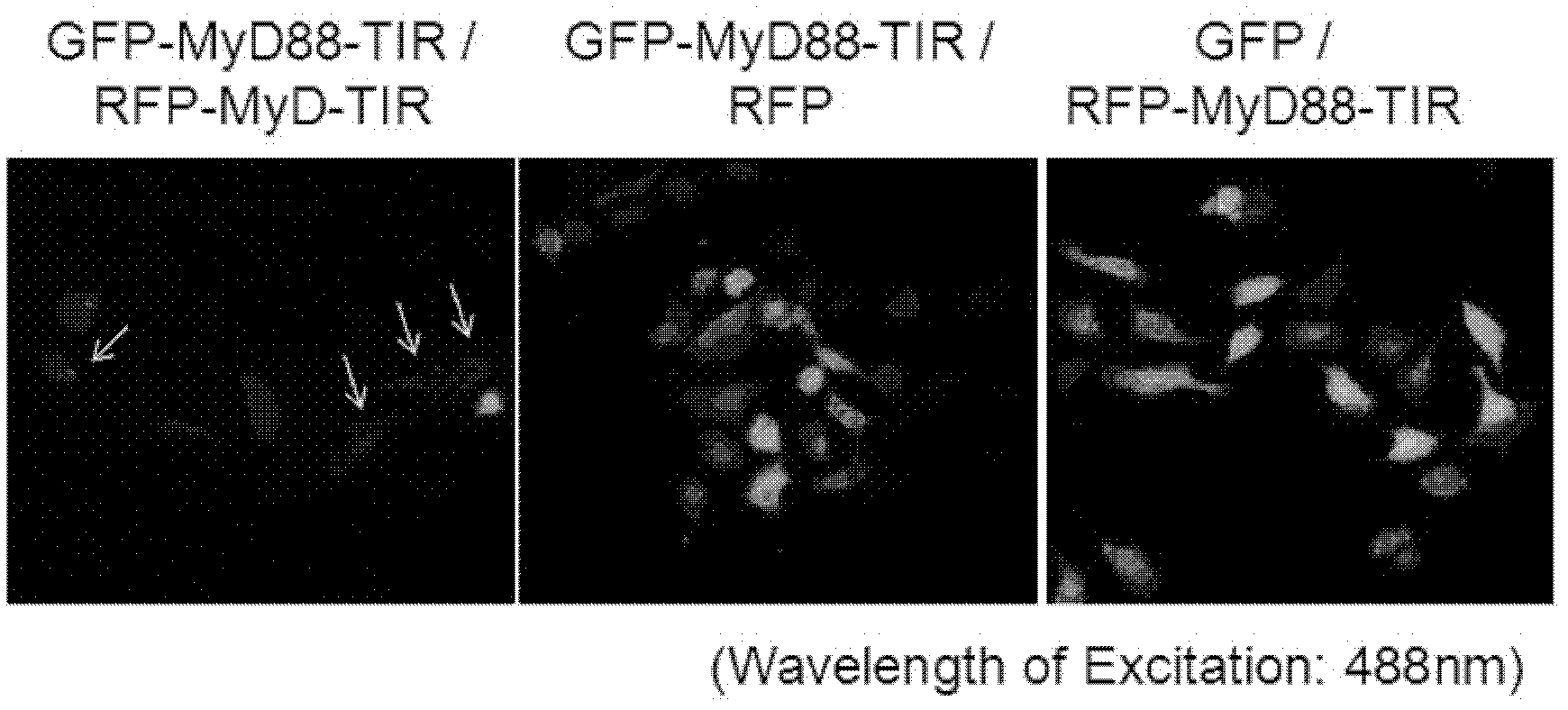
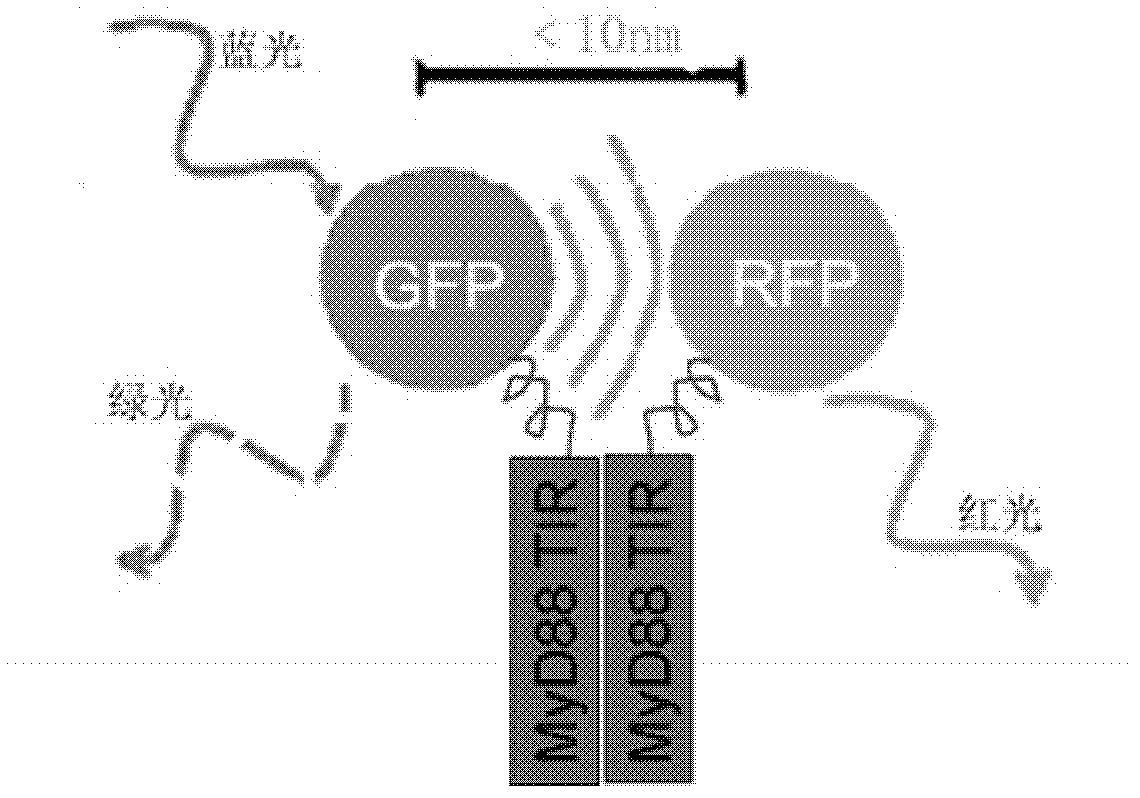
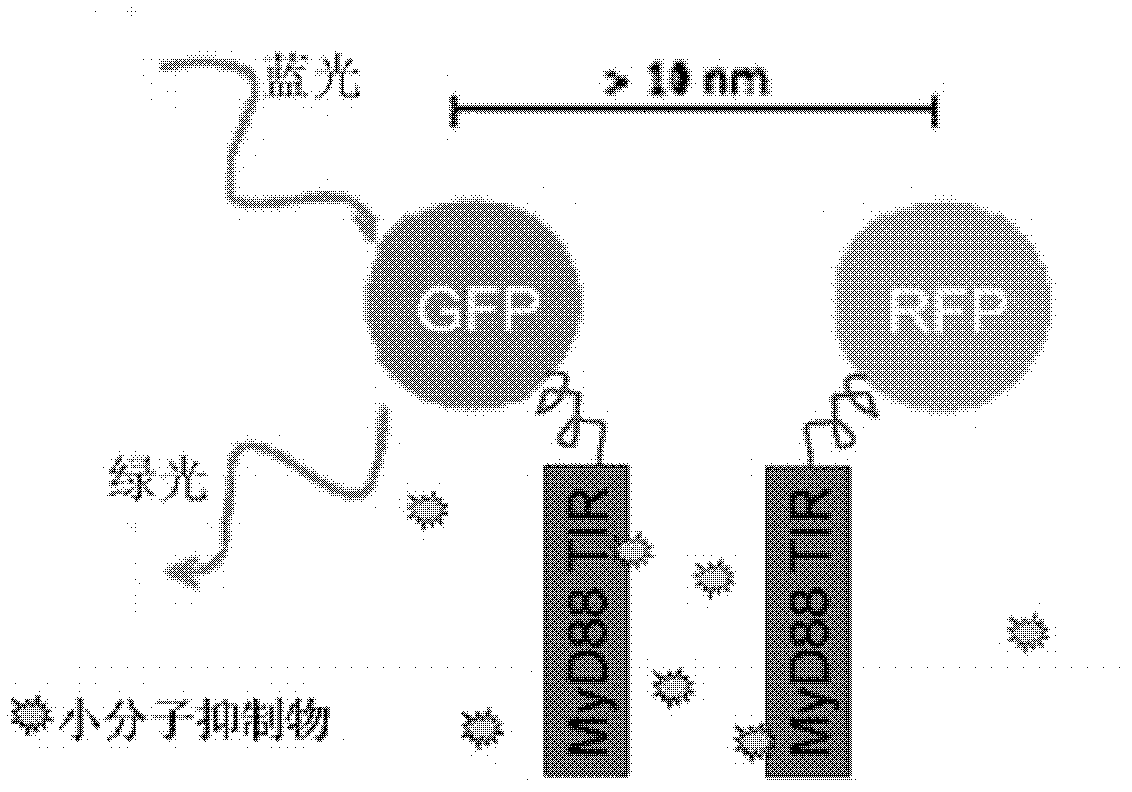
[0043] we press figure 1 The combination shown in, the constructed GFP-MyD88 TIR, RFP-MyD88 TIR, GFP, RFP plasmids were co-transfected into HeLa cells. Under 488nm excitation light, cells co-transfected with GFP-MyD88 TIR and RFP-MyD88TIR fusion proteins (left photo) emit dim green fluorescence, and even some cells emit orange light. It is proved that the green fluorescence emitted by GFP should transfer energy to RFP for the dimerization of MyD88TIR, and realize FRET. The control group co-transfected GFP-MyD88 TIR fusion protein and empty RFP plasmid (middle photo), or vice versa, co-transfected empty GFP and RFP-MyD88 TIR fusion protein plasmid (right photo), 488nm excitation light Under observation, the cells show bright green fluorescence. Description: Cells co-transfected with GFP-MyD88 TIR and RFP or co-transfected with GFP and RFP-MyD88 T...
Embodiment 2
[0045] Example 2: His-MyD88 TIR recombinant protein can interact with GST-MD88 TIR recombinant protein in vitro
[0046] There is still a problem with the in situ fluorescence model of living cells described in Example 1 above: when the FRET phenomenon in cultured cells is blocked by some added compound, we cannot yet conclude that this compound directly blocks MyD88 TIR The dimerization of MyD88 TIR indirectly affects the dimerization of MyD88 TIR, or this compound triggers some kind of intracellular signaling cascade. For this problem, we can use a His-MyD88 TIR and GST-MyD88 TIR recombinant protein interaction analysis in vitro to verify.
[0047] To this end, we constructed plasmids for the prokaryotic expression of fusion proteins GST-MyD88 TIR and His-MyD88 TIR, and induced protein expression after transformation into Escherichia coli (the recombinant fusion protein can be specifically recognized by MyD88 antibody immunoblotting, proving the correct expression of MyD88 T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com